Abstract
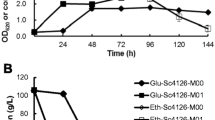
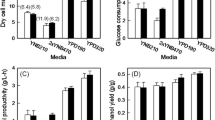
Construction of acid-tolerant strains of Saccharomyces cerevisiae is required for various bioproduction processes. We previously isolated the gene IoGAS1 from multiple stress-tolerant Issatchenkia orientalis as a gene conferring sulfuric acid resistance in S. cerevisiae, but its acid tolerance was only investigated using sulfuric acid. Here, we evaluated the growth and ethanol fermentation ability of the IoGAS1-expressing S. cerevisiae strain, B4-IoGAS1, by using various acidic reagents. B4-IoGAS1 exhibited faster growth than the control strain, B4-CON, when cultured aerobically with sulfuric, hydrochloric, formic, acetic, and lactic acids at pH below 2.4. However, the growth of B4-IoGAS1 was suppressed at pH above 2.48, irrespective of the type of acid reagents. Furthermore, B4-IoGAS1 exhibited higher performance of ethanol fermentation than B4-CON under 250 mM lactic acid condition at pH 2.37. These results demonstrate that IoGAS1 could facilitate the aerobic growth and anaerobic ethanol production under different acidic stressed conditions.



Similar content being viewed by others
References
Kavšček, M., Stražar, M., Curk, T., Natter, K., & Petrovič, U. (2015). Yeast as a cell factory: current state and perspectives. Microbial Cell Factories, 14, 94.
Thalagala, T. A. T. P., Kodama, S., Mishima, T., Isono, N., Furujyo, A., Kawasaki, Y., & Hisamatsu, M. (2009). Study on a new preparation of d-glucose rich fractions from various lignocelluloses through a two-step extraction with sulfuric acid. Journal of Applied Glycoscience, 56(1), 1–6.
Agbor, V. B., Cicek, N., Sparling, R., Berlin, A., & Levin, D. B. (2011). Biomass pretreatment: fundamentals toward application. Biotechnology Advances, 29(6), 675–685.
Rasmussen, H., Sørensen, H. R., & Meyer, A. S. (2014). Formation of degradation compounds from lignocellulosic biomass in the biorefinery: sugar reaction mechanisms. Carbohydrate Research, 385, 45–57.
Klinke, H. B., Thomsen, A. B., & Ahring, B. K. (2004). Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Applied Microbiology and Biotechnology, 66(1), 10–26.
van Maris, A. J., Abbott, D. A., Bellissimi, E., van den Brink, J., Kuyper, M., Luttik, M. A., Wisselink, H. W., Scheffers, W. A., van Dijken, J. P., & Pronk, J. T. (2006). Alcoholic fermentation of carbon sources in biomass hydrolysates by Saccharomyces cerevisiae: current status. Antonie Van Leeuwenhoek, 90(4), 391–418.
Almeida, J. R. M., Modig, T., Petersson, A., Hähn-Hägerdal, B., Lidén, G., & Gorwa-Grauslund, M. F. (2007). Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. Journal of Chemical Technology and Biotechnology, 82(4), 340–349.
Skory, C. D. (2003). Lactic acid production by Saccharomyces cerevisiae expressing a Rhizopus oryzae lactate dehydrogenase gene. Journal of Industrial Microbiology & Biotechnology, 30(1), 22–27.
Dequin, S., & Barre, P. (1994). Mixed lactic acid-alcoholic fermentation by Saccharomyces cerevisiae expressing the Lactobacillus caseil(+)-LDH. Nature Biotechnology, 12, 173–177.
Porro, D., Brambilla, L., Ranzi, B. M., Martegani, E., & Alberghina, L. (1995). Development of metabolically engineered Saccharomyces cerevisiae cells for the production of lactic acid. Biotechnology Progress, 11(3), 294–298.
Adachi, E., Torigoe, M., Sugiyama, M., Nikawa, J., & Shimizu, K. (1998). Modification of metabolic pathways of Saccharomyces cerevisiae by the expression of lactate dehydrogenase and deletion of pyruvate decarboxylase genes for the lactic acid fermentation at low pH value. Journal of Fermentation and Bioengineering, 86, 284–289.
Ishida, N., Saitoh, S., Tokuhiro, K., Nagamori, E., Matsuyama, T., Kitamoto, K., & Takahashi, H. (2005). Efficient production of L-lactic acid by metabolically engineered Saccharomyces cerevisiae with a genome-integrated l-lactate dehydrogenase gene. Applied and Environmental Microbiology, 71(4), 1964–1970.
Ishida, N., Suzuki, T., Tokuhiro, K., Nagamori, E., Onishi, T., Saitoh, S., Kitamoto, K., & Takahashi, H. (2006). D-lactic acid production by metabolically engineered Saccharomyces cerevisiae. Journal of Bioscience and Bioengineering, 101(2), 172–177.
Valli, M., Sauer, M., Branduardi, P., Borth, N., Porro, D., & Mattanovich, D. (2006). Improvement of lactic acid production in Saccharomyces cerevisiae by cell sorting for high intracellular pH. Applied and Environmental Microbiology, 72(8), 5492–5499.
Datta, R., & Henry, M. (2006). Lactic acid: recent advances in products, processes and technologies — a review. Journal of Chemical Technology and Biotechnology, 81(7), 1119–1129.
Abdel-Rahman, M. A., Tashiro, T., & Sonomoto, K. (2013). Recent advances in lactic acid production by microbial fermentation processes. Biotechnology Advances, 31(6), 877–902.
Suzuki, T., Sakamoto, T., Sugiyama, M., Ishida, N., Kambe, H., Obata, S., Kaneko, Y., Takahashi, H., & Harashima, S. (2013). Disruption of multiple genes whose deletion causes lactic-acid resistance improves lactic-acid resistance and productivity in Saccharomyces cerevisiae. Journal of Bioscience and Bioengineering, 115(5), 467–474.
Matsushika, A., Negi, K., Suzuki, T., Goshima, T., & Hoshino, T. (2016). Identification and characterization of a novel Issatchenkia orientalis GPI-anchored protein, IoGas1, required for resistance to low pH and salt stress. PLoS One, 11(9), e0161888.
Matsushika, A., Suzuki, T., Goshima, T., & Hoshino, T. (2017). Evaluation of Saccharomyces cerevisiae GAS1 with respect to its involvement in tolerance to low pH and salt stress. Journal of Bioscience and Bioengineering, 124(2), 164–170.
Aimanianda, V., Simenel, C., Garnaud, C., Clavaud, C., Tada, R., Barbin, L., Mouyna, I., Heddergott, C., Popolo, L., Ohya, Y., Delepierre, M., & Latge, J. P. (2017). The dual activity responsible for the elongation and branching of β-(1,3)-glucan in the fungal cell wall. MBio, 8(3), e00619–e00617.
Kawahata, M., Masaki, K., Fujii, T., & Iefuji, T. (2006). Yeast genes involved in response to lactic acid and acetic acid: acidic conditions caused by the organic acids in Saccharomyces cerevisiae cultures induce expression of intracellular metal metabolism genes regulated by Aft1p. FEMS Yeast Research, 6(6), 924–936.
Levin, D., & Bartlett-Heubusch, E. (1992). Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. The Journal of Cell Biology, 116(5), 1221–1229.
Yamochi, W., Tanaka, K., Nonaka, H., Maeda, A., Musha, T., & Takai, Y. (1994). Growth site localization of Rho1 small GTP-binding protein and its involvement in bud formation in Saccharomyces cerevisiae. The Journal of Cell Biology, 125(5), 1077–1093.
Ram, A. F. J., Brekelmans, S. S. C., Oehlen, L. J. W. M., & Klis, F. M. (1995). Identification of two cell cycle regulated genes affecting the β1,3-glucan content of cell walls in Saccharomyces cerevisiae. FEBS Letters, 358(2), 165–170.
Negishi, T., & Ohya, Y. (2010). The cell wall integrity checkpoint: coordination between cell wall synthesis and the cell cycle. Yeast, 27(8), 513–519.
Casal, M., Paiva, S., Queiros, O., & Soares-Silva, I. (2008). Transport of carboxylic acids in yeast. FEMS Microbiology Reviews, 32(6), 974–994.
Sauer, M., Porro, D., Mattanovich, D., & Branduardi, P. (2010). 16 years research on lactic acid production with yeast – ready for the market? Biotechnology and Genetic Engineering Reviews, 27, 229–256.
Casal, M., Paiva, S., Andrade, R. P., Gancedo, C., & Leão, C. (1999). The lactate-proton symport of Saccharomyces cerevisiae is encoded by JEN1. Journal of Bacteriology, 181(8), 2620–2623.
Pacheco, A., Talaja, G., Sá-Pessoa, J., Bessa, D., Gonçalves, M. J., Moreira, R., Paiva, S., Casal, M., & Queirós, O. (2012). Lactic acid production in Saccharomyces cerevisiae is modulated by expression of the monocarboxylate transporters Jen1 and Ady2. FEMS Yeast Research, 12(3), 375–381.
Simões, T., Mira, N. P., Fernandes, A. R., & Sá-Correia, I. (2006). The SPI1 gene, encoding a glycosylphosphatidylinositol-anchored cell wall protein, plays a prominent role in the development of yeast resistance to lipophilic weak-acid food preservatives. Applied and Environmental Microbiology, 72(11), 7168–7175.
Davis-Kaplan, S. R., Askwith, C. C., Bengtzen, A. C., Radisky, D., & Kaplan, J. (1998). Chloride is an allosteric effector of copper assembly for the yeast multicopper oxidase Fet3p: an unexpected role for intracellular chloride channels. Proceedings of the National Academy of Sciences of the United States of America, 95(23), 13641–13645.
Jennings, M. L., & Cui, J. (2008). Chloride homeostasis in Saccharomyces cerevisiae: high affinity influx, V-ATPase-dependent sequestration, and identification of a candidate Cl− sensor. Journal of General Physiology, 131(4), 379–391.
Gao, J., Feng, H., Yuan, W., Li, Y., Zhong, S., & Bai, F. (2017). Enhanced fermentative performance under stresses of multiple lignocellulose-derived inhibitors by overexpression of a typical 2-Cys peroxiredoxin from Kluyveromyces marxianus. Biotechnology for Biofuels, 10, 79.
Hasunuma, T., Sanda, T., Yamada, R., Yoshimura, K., Ishii, J., & Kondo, A. (2011). Metabolic pathway engineering based on metabolomics confers acetic and formic acid tolerance to a recombinant xylose-fermenting strain of Saccharomyces cerevisiae. Microbial Cell Factories, 10(1), 2.
Fletcher, E., Feizi, A., Bisschops, M. M. M., Hallström, B. M., Khoomrung, S., Siewers, V., & Nielsen, J. (2017). Evolutionary engineering reveals divergent paths when yeast is adapted to different acidic environments. Metabolic Engineering, 39, 19–28.
Silva, C. L., Vianna, C. R., Cadete, R. M., Santos, R. O., Gomes, F. C., Oliverira, E. C., & Rosa, C. A. (2009). Selection, growth, and chemo-sensory evaluation of flocculent starter culture strains of Saccharomyces cerevisiae in the large-scale production of traditional Brazilian cachaça. International Journal of Food Microbiology, 131(2–3), 203–210.
Sharma, S. K., Kalra, K. L., & Grewal, H. S. (2002). Fermentation of enzymatically saccharified sunflower stalks for ethanol production and its scale up. Bioresource Technology, 85(1), 31–33.
Acknowledgments
We thank Dr. Zheng Xiahong for technical support. We would like to thank Editage (www.editage.com) for English language editing.
Funding
This study was supported by Basic Research Funding of the National Institute of Advanced Industrial Science and Technology (AIST).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplemental Fig 1
Growth curve of the B4-CON and the B4-IoGAS1 strains with formic and acetic acids containing sulfuric acid. The B4-CON (red) and the B4-IoGAS1 (blue) strains were cultured aerobically in SCD medium containing 7 mM sulfuric acid and various concentration of formic (A) and acetic (B) acids for evaluating the growth characteristics for 96 h. Data shown are the mean ± SD (n = 3) (PDF 67 kb)
ESM 2
(PDF 73 kb)
Rights and permissions
About this article
Cite this article
Wada, K., Fujii, T., Akita, H. et al. IoGAS1, a GPI-Anchored Protein Derived from Issatchenkia orientalis, Confers Tolerance of Saccharomyces cerevisiae to Multiple Acids. Appl Biochem Biotechnol 190, 1349–1359 (2020). https://doi.org/10.1007/s12010-019-03187-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03187-8