Abstract
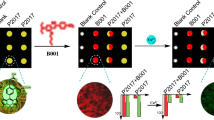
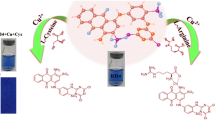
A new colorimetric chemosensor for naked-eye detection and determination of cysteine (Cys) based on indicator displacement assay (IDA) was designed using 1-(2-pyridylazo)-2-naphthol (PAN). The indicator exchange occurred between PAN and Cys by the addition of Cys to the Cu(PAN)2 complex, which is accomplished by an immediate visible color change from magenta to yellow, in the solution phase and paper-based test strips. The proposed method exhibits 0.35 μmol L−1 detection limit and good linearity in the range of 2.25–42.91 μmol L−1. Paper test strips presented a detection limit of 38.0 μmol L−1, fabricating an easy to use test kit for compatible “in-the-field” detection of Cys. The computer image analysis of the paper test strips, obtained from the CMYK color analysis system, showed a linear increase in Y (yellow) intensity with enhancement in the Cys concentration of 50.0–550.0 μmol L−1. Additionally, the absorption and color change obtained in this chemosensor operate as an “INHIBIT” logic gate considering Cu2+ and Cys as inputs. Eventually, based on such a fast, reversible, and reproducible signal, a molecular-scale sequential memory unit was designed displaying “Writing–Reading–Erasing–Reading” and “Multi-Write” behavior. The developed chemosensor presented a satisfactory repeatability, intermediate precision, and successful application for the selective determination of Cys in human biological fluids.

.













Similar content being viewed by others
References
van de Rest, O., van der Zwaluw, N. L., & de Groot, L. C. (2013). Literature review on the role of dietary protein and amino acids in cognitive functioning and cognitive decline. Amino Acids, 45(5), 1035–1045.
Wahl, O., & Holzgrabe, U. (2016). Amino acid analysis for pharmacopoeial purposes. Talanta, 154, 150–163.
Hashemi, H. S., Nezamzadeh-Ejhieh, A., & Karimi-Shamsabadi, M. (2016). A novel cysteine sensor based on modification of carbon paste electrode by Fe(II)-exchanged zeolite X nanoparticles. Materials Science & Engineering. C, Materials for Biological Applications, 58, 286–293.
Wei, X., Qi, L., Tan, J., Liu, R., & Wang, F. (2010). A colorimetric sensor for determination of cysteine by carboxymethyl cellulose-functionalized gold nanoparticles. Analytica Chimica Acta, 671(1-2), 80–84.
Borase, H. P., Patil, C. D., Salunkhe, R. B., Suryawanshi, R. K., Kim, B. S., Bapat, V. A., & Patil, S. V. (2015). Bio-functionalized silver nanoparticles: a novel colorimetric probe for cysteine detection. Applied Biochemistry and Biotechnology, 175(7), 3479–3493.
Amarnath, K., Amarnath, V., Amarnath, K., Valentine, H. L., & Valentine, W. M. (2003). A specific HPLC-UV method for the determination of cysteine and related aminothiols in biological samples. Talanta, 60(6), 1229–1238.
Inoue, T., & Kirchhoff, J. R. (2002). Determination of thiols by capillary electrophoresis with amperometric detection at a coenzyme pyrroloquinoline quinone modified electrode. Analytical Chemistry, 74(6), 1349–1354.
Tavallali, H., & Massoumi, A. (1998). Simultaneous kinetic spectrophotometric determination of vanadium(V) and iron(III). Talanta, 47(2), 479–485.
Türkekul, K., Üzer, A., Can, Z., Erçağ, E., & Apak, R. (2019). Colorimetric sensing of the insensitive energetic material 3-nitro-1,2,4-triazol-5-one (NTO) using l-cysteine stabilized gold nanoparticles and copper(II). Analytical Letters, 1-13.
Xiao, W., Hu, H., & Huang, J. (2012). Colorimetric detection of cysteine by surface functionalization of natural cellulose substance. Sensors and Actuators B: Chemical, 171-172, 878–885.
Duan, L., Xu, Y., Qian, X., Wang, F., Liu, J., & Cheng, T. (2008). Highly selective fluorescent chemosensor with red shift for cysteine in buffer solution and its bioimage: symmetrical naphthalimide aldehyde. Tetrahedron Letters, 49(47), 6624–6627.
Lee, S. A., Lee, J. J., Shin, J. W., Min, K. S., & Kim, C. (2015). A colorimetric chemosensor for the sequential detection of copper(II) and cysteine. Dyes and Pigments, 116, 131–138.
Tavallali, H., Deilamy-Rad, G., Parhami, A., & Mousavi, S. Z. (2013). A novel development of dithizone as a dual-analyte colorimetric chemosensor: detection and determination of cyanide and cobalt (II) ions in dimethyl sulfoxide/water media with biological applications. Journal of Photochemistry and Photobiology. B, 125, 121–130.
Tavallali, H., Baezzat, M.-R., Deilamy-Rad, G., Parhami, A., & Hasanli, N. (2015). An ultrasensitive and highly selective fluorescent and colorimetric chemosensor for citrate ions based on rhodamine B and its application as the first molecular security keypad lock based on phosphomolybdic acid and citrate inputs. Journal of Luminescence, 160, 328–336.
de Silva, P. A., Gunaratne, N. H. Q., & McCoy, C. P. (1993). A molecular photoionic AND gate based on fluorescent signalling. Nature., 364(6432), 42–44.
Tang, L., Wu, D., Wen, X., Dai, X., & Zhong, K. (2014). A novel carbazole-based ratiometric fluorescent sensor for Zn2+ recognition through excimer formation and application of the resultant complex for colorimetric recognition of oxalate through IDAs. Tetrahedron, 70(47), 9118–9124.
Xu, X., Zhang, N., Brown, G. M., Thundat, T. G., & Ji, H.-F. (2017). Ultrasensitive detection of Cu2+ using a microcantilever sensor modified with L-cysteine self-assembled monolayer. Applied Biochemistry and Biotechnology, 183(2), 555–565.
Zhang, D. (2009). Highly selective colorimetric detection of cysteine and homocysteine in water through a direct displacement approach. Inorganic Chemistry Communications, 12(12), 1255–1258.
Arvand, M., Abolghasemi, S., & Zanjanchi, M. A. (2007). Simultaneous determination of zinc and copper(II) with 1-(2-pyridylazo)2-naphthol in micellar media by spectrophotometric H-point standard addition method. Journal of Analytical Chemistry, 62(4), 342–347.
Cheng, K. L. (1958). Complexometric titration of copper and other metals in mixture. 1-(2-Pyridylazo)-2-naphthol (dye) as indicator. Analytical Chemistry, 30(2), 243–245.
Zhang, D., & Jin, W. (2012). Highly selective and sensitive colorimetric probe for hydrogen sulfide by a copper (II) complex of azo-dye based on chemosensing ensemble approach. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy., 90, 35–39.
Shah, P., Zhu, X., & Li, C.-Z. (2013). Development of paper-based analytical kit for point-of-care testing. Expert Review of Molecular Diagnostics, 13(1), 83–91.
Martinez, A. W., Phillips, S. T., Whitesides, G. M., & Carrilho, E. (2010). Diagnostics for the developing world: microfluidic paper-based analytical devices. Analytical Chemistry, 82(1), 3–10.
Gelsema, W. J., de Ligny, C. L., Remijnse, A. G., & Blijleven, H. A. (1966). pH-Measurements in alcohol-water mixtures, using aqueous standard buffer solutions for calibration. Recueil des Travaux Chimiques des Pays-Bas, 85(7), 647–660.
Gelsema, W. J., de Ligny, C. L., & Blijleven, H. A. (1967). pH-measurements in alcohol-water mixtures. On the use of aqueous standard buffer solutions for calibration. Recueil des Travaux Chimiques des Pays-Bas, 86(8), 852–864.
Katrusiak, A. E., Paterson, P. G., Kamencic, H., Shoker, A., & Lyon, A. W. (2001). Pre-column derivatization high-performance liquid chromatographic method for determination of cysteine, cysteinyl–glycine, homocysteine and glutathione in plasma and cell extracts. Journal of Chromatography. B, Biomedical Sciences and Applications, 758(2), 207–212.
Chen, W., Zhao, Y., Seefeldt, T., & Guan, X. (2008). Determination of thiols and disulfides via HPLC quantification of 5-thio-2-nitrobenzoic acid. Journal of Pharmaceutical and Biomedical Analysis, 48(5), 1375–1380.
Berthon, G. (1995). Critical evaluation of the stability constants of metal complexes of amino acids with polar side chains (technical report). Pure and Applied Chemistry, pp. 1117.
Remelli, M., Peana, M., Medici, S., Delogu, L. G., & Zoroddu, M. A. (2013). Interaction of divalent cations with peptide fragments from Parkinson’s disease genes. Dalton Transactions, 42(17), 5964–5974.
Dokken, K. M., Parsons, J. G., McClure, J., & Gardea-Torresdey, J. L. (2009). Synthesis and structural analysis of copper(II) cysteine complexes. Inorganica Chimica Acta, 362(2), 395–401.
Firdaus, M. L., Alwi, W., Trinoveldi, F., Rahayu, I., Rahmidar, L., & Warsito, K. (2014). Determination of chromium and iron using digital image-based colorimetry. Procedia Environmental Sciences, 20, 298–304.
Apyari, V. V., Dmitrienko, S. G., & Zolotov, Y. A. (2011). Analytical possibilities of digital colorimetry: determination of nitrite using polyurethane foam. Moscow University Chemistry Bulletin, 66(1), 32–37.
Gonzalez, R. C., & Woods, R. E. (2006). Digital image processing (3rd ed.). New Jersey: Prentice-Hall.
Stroebel, L. and Zakia, R. D. (1993). The focal encyclopedia of photography. ed. Focal Press.
Ooi, S. i., Carter, D., & Fernando, Q. (1967). The structure of a chelate of copper(II) with 1-(2-pyridylazo)-2-naphthol. Chemical Communications (London), (24), 1301–1302.
Szabó, L., Herman, K., Mircescu, N. E., Fălămaş, A., Leopold, L. F., Leopold, N., Buzumurgă, C., & Chiş, V. (2012). SERS and DFT investigation of 1-(2-pyridylazo)-2-naphthol and its metal complexes with Al(III), Mn(II), Fe(III), Cu(II), Zn(II) and Pb(II). Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 93, 266–273.
Walsh, M. J., Goodnow, S. D., Vezeau, G. E., Richter, L. V., & Ahner, B. A. (2015). Cysteine enhances bioavailability of copper to marine phytoplankton. Environmental Science & Technology, 49(20), 12145–12152.
Berg, J. M., Tymoczko, J. L., & Stryer, L. (2002). Biochemistry (5th ed.). W.H. Freeman.
Skoog, D. A., West, D. M., Holler, F. J. and Crouch, S. R. (2013). Fundamentals of analytical chemistry. ed. Cengage Learning.
de Silva, A. P., Uchiyama, S., Vance, T. P., & Wannalerse, B. (2007). A supramolecular chemistry basis for molecular logic and computation. Coordination Chemistry Reviews, 251(13–14), 1623–1632.
Lu, W., Zhang, M., Liu, K., Fan, B., Xia, Z., & Jiang, L. (2011). A fluoride-selective colorimetric and fluorescent chemosensor and its use for the design of molecular-scale logic devices. Sensors and Actuators B: Chemical, 160(1), 1005–1010.
Rafii, M., Elango, R., Courtney-Martin, G., House, J. D., Fisher, L., & Pencharz, P. B. (2007). High-throughput and simultaneous measurement of homocysteine and cysteine in human plasma and urine by liquid chromatography–electrospray tandem mass spectrometry. Analytical Biochemistry, 371(1), 71–81.
Brigham, M. P., Stein, W. H., & Moore, S. The concentrations of cysteine and cystine in human blood plasma. The Journal of Clinical Investigation, 39(11), 1633–1638.
Kuśmierek, K., Głowacki, R., & Bald, E. (2006). Analysis of urine for cysteine, cysteinylglycine, and homocysteine by high-performance liquid chromatography. Analytical and Bioanalytical Chemistry, 385(5), 855–860.
Xu, X., Qiao, J., Li, N., Qi, L., & Zhang, S. (2015). Fluorescent probe for turn-on sensing of L-cysteine by ensemble of AuNCs and polymer protected AuNPs. Analytica Chimica Acta, 879, 97–103.
Ellman, G. L. (1959). Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics, 82(1), 70–77.
Burtis, C. A. and Bruns, D. E. (2014). Tietz fundamentals of clinical chemistry and molecular diagnostics—e-book. ed. Elsevier Health Sciences.
Lu, J., Sun, C., Chen, W., Ma, H., Shi, W., & Li, X. (2011). Determination of non-protein cysteine in human serum by a designed BODIPY-based fluorescent probe. Talanta, 83(3), 1050–1056.
Anderson, R. L. (1987). Practical statistics for analytical chemists. ed. Van Nostrand Reinhold.
Xue, S., Ding, S., Zhai, Q., Zhang, H., & Feng, G. (2015). A readily available colorimetric and near-infrared fluorescent turn-on probe for rapid and selective detection of cysteine in living cells. Biosensors & Bioelectronics, 68, 316–321.
Farhadi, K., Forough, M., Pourhossein, A., & Molaei, R. (2014). Highly sensitive and selective colorimetric probe for determination of l-cysteine in aqueous media based on Ag/Pd bimetallic nanoparticles. Sensors and Actuators B: Chemical, 202, 993–1001.
Khajehsharifi, H., & Sheini, A. (2014). A selective naked-eye detection and determination of cysteine using an indicator-displacement assay in urine sample. Sensors and Actuators B: Chemical, 199, 457–462.
Funding
The authors wish to acknowledge the support of this work by Payame Noor University Research Council.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• A novel IDA colorimetric chemosensor was developed for the determination of cysteine.
• The paper-based chemosensor was designed according to the IDA mechanism.
• The prepared test kit was analyzed using a digital camera and the CMYK software.
• The paper strips can be applied in clinical analysis in combination with a camera.
• The color changes in aqueous and paper-based chemosensors are reversible.
Rights and permissions
About this article
Cite this article
Deilamy-Rad, G., Asghari, K. & Tavallali, H. Development of a Reversible Indicator Displacement Assay Based on the 1-(2-Pyridylazo)-2-naphthol for Colorimetric Determination of Cysteine in Biological Samples and Its Application to Constructing the Paper Test Strips and a Molecular-Scale Set/Reset Memorized Device. Appl Biochem Biotechnol 192, 85–102 (2020). https://doi.org/10.1007/s12010-019-03165-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03165-0