Abstract
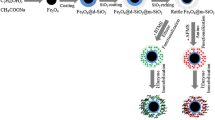
In this work, novel silica hybrid magnetic particles with biocompatible surface were designed as a support for enzyme immobilization, and the immobilized chymotrypsin (CT) performance was clarified as a model biocatalyst. CT is used in food technology for drink clarification and protein hydrolysis. The enzyme was directly immobilized onto polydopamine-grafted magnetic silica particles (MNP@SiO2@PDA-CT) via the Schiff base reaction. Immobilized enzyme had broadened for both pH and temperature profiles compared with the native CT. The MNP@SiO2@PDA-CT system also improved in thermostability compared with the native enzyme. The immobilized CT was operated in a continuous enzyme reactor (CER) for the hydrolysis of different proteins (i.e., cytochrome c (Cyt c), human serum albumin (HSA), human immunoglobulin G (HIgG), and lysozyme (Lys)). The peptide synthesis rate was shown to decrease as a function of increasing flow rate. The catalytic activity of the CER remained stable for 6.0 h in a continuous operation period; thus, the presented method may increase applicability in the food technology area. The immobilized CT in the CER showed a good hydrolysis performance for all the tested model proteins. The peptides hydrolyzed from the tested proteins were analyzed with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-ToF-MS). Results show that the MNP@SiO2@PDA-CT system also permits for the applicability in the area of proteomic research.








Similar content being viewed by others
References
Arica, M. Y., Soydogan, H., & Bayramoglu, G. (2010). Reversible immobilization of Candida rugosa lipase on fibrous polymer grafted and sulfonated p(HEMA/EGDMA) beads. Bioprocess and Biosystems Engineering, 33, 227–236.
Bayramoglu, G., Kayili, H. M., Oztekin, M., Salih, B., & Arica, M. Y. (2020). Hydrophilic spacer-arm containing magnetic nanoparticles for immobilization of proteinase K: employment for speciation of proteins for mass spectrometry-based analysis. Talanta, 206, 120218.
Dos Santos, J. C. S., Rueda, N., Barbosa, O., Fernandez-Sanchez, J. F., Medina-Castillo, A. L., Ramon-Marquez, T., Arias-Martos, M. C., Millan-Linares, M. C., Pedroche, J., Del Mar Yust, M., Goncalves, L. R. B., & Fernandez-Lafuente, R. (2015). Characterization of supports activated with divinyl sulfone as a tool to immobilize and stabilize enzymes via multipoint covalent attachment application to chymotrypsin. RSC Advances, 5, 20639.
Ulu, A., Noma, S. A. A., Koytepe, S., & Ates, B. (2019). Chloro-modified magnetic fe3o4@mcm-41 core–shell nanoparticles for l-asparaginase immobilization with improved catalytic activity, reusability, and storage stability. Applied Biochemistry and Biotechnology, 187, 938–956.
Oh, H., & Lee, K. H. (2013). Activity and stability of immobilized enzyme on silk sericin bead. International Journal of Industrial Entomology, 27, 329–332.
Bayramoglu, G., Akbulut, A., & Arica, M. Y. (2013). Immobilization of tyrosinase on modified diatom biosilica: enzymatic removal of phenolic compounds from aqueous solution. Journal of Hazardous Materials, 244–245, 528–536.
Kumaria, S., Chauhana, G. S., Ahn, J.-H., & Reddy, N. S. (2016). Bio-waste derived dialdehyde cellulose ethers as supports for chymotrypsin immobilization. International Journal of Biological Macromolecules, 85, 227–237.
Goncalves, M. C. P., Kieckbusch, T. G., Perna, R. F., Fujimoto, J. T., Morales, S. A. V., & Romanelli, J. P. (2019). Trends on enzyme immobilization researches based on bibliometric analysis. Process Biochemistry, 76, 95–110.
Azevedo, R. D. S., Amaral, I. P. G., Ferreira, A. C. M., Esposito, T. S., & Bezerra, R. S. (2018). Use of fish trypsin immobilized onto magnetic-chitosan composite as a new tool to detect anti-nutrients in aqua-feeds. Food Chemistry, 257, 302–330.
Gennari, A., Mobayed, F. H., Nervis, B. D. R., Benvenutti, E. V., Nicolodi, S., da Silveira, N. P., Volpato, G., & de Souza, V. C. F. (2019). Immobilization of β-galactosidases on magnetic nanocellulose: textural, morphological, magnetic, and catalytic properties. Biomacromolecules, 20, 2315–2326.
Arica, M. Y. (2000). Epoxy-derived phema membrane for use bioactive macromolecules immobilization: covalently bound urease in a continuous model system. Journal of Applied Polymer Science, 77, 2000–2008.
Arica, T. A., Kuman, M., Gercel, O., & Ayas, E. (2019). Poly(dopamine) grafted bio-silica composite with tetraethylenepentamine ligands for enhanced adsorption of pollutants. Chemical Engineering Research and Design, 141, 317–327.
Siddiqui, I., & Husain, Q. (2019). Stabilization of polydopamine modified silver nanoparticles bound trypsin: insights on protein hydrolysis. Colloids and Surface B, 173, 733–741.
Zhang, L., Guo, X., Song, Y., Tan, N., Cheng, P. I., Xiang, J., & Du, W. (2018). Bioadhesive immobilize agarase on magnetic ferriferous by polydopamine. Materials Science and Engineering: C, 93, 218–225.
Messersmith, P. B., Lee, H., Dellatore, S. M., & Miller, W. M. (2007). Mussel-inspired surface chemistry for multifunctional coatings. Science, 318, 426–430.
Yoshimoto, M., Yamada, J., Baba, M., & Walde, P. (2016). Enhanced heat stability of chymotrypsin through single-enzyme confinement in attoliter liposomes. Chembiochemistry, 17(13), 1221–1224.
Bayramoglu, G., & Arica, M. Y. (2008). Enzymatic removal of phenol and p-chlorophenol in enzyme reactor: horseradish peroxidase immobilized on magnetic beads. Journal of Hazardous Materials, 156(1-3), 148–155.
Tang, A., Zhang, Y., Wei, T., Wu, J., Li, Q., & Liu, Y. (2019). Immobilization of candida cylindracea lipase by covalent attachment on glu-modified bentonite. Applied Biochemistry and Biotechnology, 187(3), 870–883.
Xie, X., Luo, P., Han, J., Chen, T., Wang, Y., Cai, Y., & Liud, Q. (2019). Horseradish peroxidase immobilized on the magnetic composite microspheres for high catalytic ability and operational stability. Enzyme and Microbial Technology, 122, 26–35.
Zhao, J.-F., Lin, J.-P., Yang, L.-R., & Wu, M.-B. (2019). Enhanced performance of rhizopus oryzae, lipase by reasonable immobilization on magnetic nanoparticles and its application in synthesis 1,3-diacyglycerol. Applied Biochemistry and Biotechnology, 188(3), 677–689.
Fu, M., Xing, J., & Ge, Z. (2019). Preparation of laccase-loaded magnetic nanoflowers and their recycling for efficient degradation of bisphenol A. The Science of the Total Environment, 651, 2857–2865.
Mei, S., Shi, J., Zhang, S., Wang, Y., Wu, Y., Jiang, Z., & Wu, H. (2019). Nanoporous phyllosilicate assemblies for enzyme immobilization. ACS Applied Biomaterials, 2, 777–786.
Banerjee, R., Katsenovich, Y., Lagos, L., McIintosh, M., Zhang, X., & Li, C.-Z. (2010). Nanomedicine: magnetic nanoparticles and their biomedical applications. Current Medicinal Chemistry, 17, 3120–3141.
Talekar, S., Ghodake, V., Ghotage, T., Rathod, P., Deshmukh, P., Nadar, S., Mulla, M., & Ladole, M. (2012). Novel magnetic cross-linked enzyme aggregates (magnetic CLEAs) of alpha amylase. Bioresource Technology, 123, 542–547.
Banerjee, R., Katsenovich, Y., Lagos, L., Senn, M., Naja, M., Balsamo, V., Pannell, K. H., & Li, C.-Z. (2010). Functional magnetic nano-shells integrated nanosensor for trace analysis of environmental uranium contamination. Electrochimica Acta, 55, 7897–7902.
Bayramoglu, G., Ozalp, V. C., & Arica, M. Y. (2014). Magnetic polymeric beads functionalized with different mixed-mode ligands for reversible immobilization of trypsin. Industrial and Engineering Chemistry Research, 53, 132–140.
Bayramoglu, G., Salih, B., Akbulut, A., & Arica, M. Y. (2019). Biodegradation of Cibacron Blue 3GA by insolubilized laccase and identification of enzymatic byproduct using MALDI-TOF-MS: toxicity assessment studies by Daphnia magna and Chlorella vulgaris. Ecotoxicolology and Environmental Safety, 170, 453–460.
Arica, M. Y., Salih, B., Celikbicak, O., & Bayramoglu, G. (2017). Immobilization of laccase on the fibrous polymer-grafted film and study of textile dye degradation by MALDI–ToF-MS. Chemical Engineering Research and Design, 128, 107–119.
Schuabb, V., Winter, R., & Czeslik, C. (2016). Improved activity of chymotrypsin on silica particles – a high-pressure stopped-flow study. Biophysical Chemistry, 218, 1–6.
Efremova, M. V., Veselov, M. M., Barulin, A. V., Gribanovsky, S. L., Le-Deygen, I. M., Uporov, I. V., Kudryashova, E. V., Sokolsky-Papkov, M., Majouga, A. G., Golovin, Y. I., Kabanov, A. V., & Klyachkoin, N. L. (2018). Situ observation of chymotrypsin catalytic activity change actuated by nonheating low- frequency magnetic field. ACS Nano, 12(4), 3190–3199.
Bahamondes, C., & Illanes, A. (2018). Effect of internal diffusional restrictions on the selectivity of chymotrypsin in a series-parallel reaction of peptide synthesis. Process Biochemistry, 68, 117–120.
Weltz, J. S., Kienle, D. F., Schwartz, D. K., & Kaa, J. L. (2019). Dramatic increase in catalytic performance of immobilized lipases by their stabilization on polymer brush supports. ACS Catalysis, 9, 4992–5001.
Hedstrom, L., Farr-Jones, S., Kettner, C. A., & Rutter, W. J. (1994). Converting trypsin to chymotrypsin: ground-state binding does not determine substrate specificity. Biochemistry, 33(29), 8764–8769.
Wong, D. E., Senecal, K. J., & Goddard, J. M. (2017). Immobilization of chymotrypsin on hierarchical nylon 6,6 nanofiber improves enzyme performance. Colloids and Surfaces, B: Biointerfaces, 154, 270–278.
Lee, B., Kim, B. C., Chang, M. S., Kim, H. S., Na, H. B., Park, Y. I., Lee, J., Hyeon, T., Lee, H., Lee, S.-W., & Kim, J. (2016). Efficient protein digestion using highly-stable and reproducible trypsin coatings on magnetic nanofibers. Chemical Engineering Journal, 288, 770–777.
Wouters, M. A., Liu, K., Riek, P., & Husain, A. A. (2003). Despecialization step underlying evolution of a family of serine proteases. Molecular Cell, 12(2), 343–354.
Plyushchenko, A. V., Borovikova, L. N., & Pisarev, O. A. (2018). Proteolytic activity of chymotrypsin immobilized on selenium nanoparticles. Applied Biochemistry and Microbiolology, 54, 375–378.
Bahamondes, C., Alvaro, G., Wilson, L., & Illanes, A. (2017). Effect of enzyme load and catalyst particle size on the diffusional restrictions in reactions of synthesis and hydrolysis catalyzed by chymotrypsin immobilized into glyoxal-agarose. Process Biochemistry, 53, 172–179.
Li, D. -F., Ding, H. -C., & Zhou, T. (2013). Covalent, immobilization of mixed proteases, trypsin and, chymotrypsin onto modified polyvinyl chloride microspheres. Journal of Agricultural and Food Chemistry, 61, 10447–10453.
Faure, N. E., Halling, P. J., & Wimperis, S. (2014). A solid-state NMR study of the immobilization of chymotrypsin on mesoporous silica. Journal of Physical Chemistry C, 118, 1042–1048.
Adriano, W. S., Mendonca, D. B., Rodrigues, D. S., Mammarella, E. J., & Giordano, R. L. C. (2008). Improving the properties of chitosan as support for the covalent multipoint of immobilization chymotrypsin. Biomacromolecules, 9(8), 2170–2179.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Meller, K., Pomastowski, P., Grzywinski, D., Szumski, M., & Buszewski, B. (2016). Preparation and evaluation of dual-enzyme micro-reactor with co-immobilized trypsin and chymotrypsin. Journal of Chromatography A, 1440, 45–54.
Bayramoglu, G., & Arica, M. Y. (2014). Activity and stability of urease entrapped in thermosensitive p(isopropylacrylamide-co-poly(ethyleneglycol)-methacrylate) hydrogel. Bioprocess and Biosystems Engineering, 37(2), 235–243.
Wang, D., Ding, W., Zhou, K., Guo, S., Zhang, Q., & Haddleton, D. M. (2018). Coating titania nanoparticles with epoxy-containing catechol polymers via Cu(0)-living radical polymerization as intelligent enzyme carriers. Biomacromolecules, 19(7), 2979–2990.
Cloete, W. J., Hayward, S., Swart, P., & Klumperman, B. (2019). Degradation of proteins and starch by combined immobilization of protease, amylase and galactosidase on a single electrospun nanofibrous membrane. Molecules, 24, 508.
Chan, Y. W., Acquah, C., Obeng, E. M., Dullah, E. C., Jeevanandam, J., & Ongkudon, C. M. (2019). Parametric study of immobilized cellulase-polymethacrylate particle for the hydrolysis of carboxymethyl cellulose. Biochimie, 157, 204–212.
Gabriele, F., Spreti, N., Del Giacco, T., Germani, R., & Tiecco, M. (2018). Effect of surfactant structure on the super activity of Candida rugosa lipase. Langmuir, 34(38), 11510–11517.
Li, L., Dyer, P. W., & Greenwell, H. C. (2018). Biodiesel production via trans-esterification using pseudomonas cepacia immobilized on cellulosic polyurethane. ACS Omega, 3(6), 6804–6811.
Arica, M. Y., & Bayramoglu, G. (2004). Polyethyleneimine-grafted poly(hydroxyethyl methacrylate-co-glycidyl methacrylate) membranes for reversible glucose oxidase immobilization. Biochemical Engineering Journal, 20, 73–77.
Zhao, J., Yu, C. -L., Fang, W., Lin, J. -D., Chen, G., & Wang, X. -Q. (2019). Spectroscopic and mechanistic analysis of the interaction between jack bean urease and polypseudorotaxane fabricated with bis-thiolated poly(ethylene glycol) and cyclodextrin. Colloid and Surfaces B, 176, 276–287.
Funding
This work was supported by the Ministry of Development–Republic of Turkey with the project number: FAY-2015-7201.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 736 kb)
Rights and permissions
About this article
Cite this article
Bayramoglu, G., Salih, B. & Arica, M.Y. Catalytic Activity of Immobilized Chymotrypsin on Hybrid Silica-Magnetic Biocompatible Particles and Its Application in Peptide Synthesis. Appl Biochem Biotechnol 190, 1224–1241 (2020). https://doi.org/10.1007/s12010-019-03158-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03158-z