Abstract
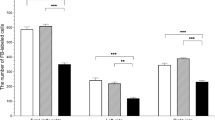
Endometritis is an inflammation of the endometrium associated with bacterial infection. The pathogenesis of endometritis in cows is still not completely understood. The combined analysis of the markers of inflammation and oxidative stress has contributed to a better understanding of disease mechanisms, but is still unexplored in uterine disorders. Moreover, research provides evidence about an important role of the vagus nerve in regulating the innate immune function through the cholinergic anti-inflammatory pathway in response to bacterial infections. This new pathway has demonstrated a critical role in controlling the inflammatory system. The aim of this study was to evaluate the activity of cholinesterase in total blood, lymphocytes, and serum of dairy cows with clinical and subclinical endometritis. Sixty-one Holstein cows, between 30 and 45 days in milk, were classified into 3 groups of animals: presenting clinical endometritis (n = 22), subclinical endometritis (n = 17), and healthy (n = 22). Mean leukocyte counts did not differ among groups, but the neutrophil number was significantly higher in cows with clinical endometritis than those in healthy animals. Also, serum concentration of interleukin-1beta (pg/mL) was significantly higher in cows with endometritis. The activity of acetylcholinesterase in blood and lymphocytes increased in both groups with endometritis. Animals with endometritis presented an increase in lipid peroxidation, but the antioxidant enzyme activity (catalase levels) was higher in endometritis groups than in normal cows. In conclusion, the inflammatory process of clinical and subclinical endometritis leads to systemic lipid peroxidation despite the compensatory increase of the antioxidant enzyme. These data also provide evidence of an important role of the cholinergic pathway in regulating dairy cows with clinical and subclinical endometritis.



Similar content being viewed by others
References
Sheldon, I. M., Cronin, J. G., & Bromfield, J. J. (2018). Tolerance and innate immunity shape the development of postpartum uterine disease and the impact of endometritis in dairy cattle. Annual Review of Animal Biosciences., 7(1), 361–384. https://doi.org/10.1146/annurev-animal-020518-115227.
Sheldon, I. M., Lewis, G. S., LeBlanc, S., & Gilbert, R. O. (2006). Defining postpartum uterine disease in cattle. Theriogenology., 65(8), 1516–1530. https://doi.org/10.1016/j.theriogenology.2005.08.021.
Sheldon, I. M., & Owens, S. E. (2017). Postpartum uterine infection and endometritis in dairy cattle. Animal Reproduction, 14(3), 622–629. https://doi.org/10.21451/1984-3143-ar1006.
Azawi, O. I. (2008). Postpartum uterine infection in cattle. Animal Reproduction Science. https://doi.org/10.1016/j.anireprosci.2008.01.010.
Sheldon, M., Cronin, J., Goetze, L., Donofrio, G., & Schuberth, H. (2009). Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biology of reproduction. https://doi.org/10.1095/biolreprod.109.077370.
Wagener, K., Gabler, C., & Drillich, M. (2017). A review of the ongoing discussion about definition, diagnosis and pathomechanism of subclinical endometritis in dairy cows. Theriogenology. https://doi.org/10.1016/j.theriogenology.2017.02.005.
da Silva, A. S., de Andrade Neto, O. A. S., Costa, M. M., Wolkmer, P., Mazzantti, C. M., Santurio, J. M., … Monteiro, S. G. (2010). Trypanosomosis in equines in southern Brazil. Acta Scientiae Veterinariae, 38(2).
Kasimanickam, R., Duffield, T. F., Foster, R. A., Gartley, C. J., Leslie, K. E., Walton, J. S., & Johnson, W. H. (2004). Endometrial cytology and ultrasonography for the detection of subclinical endometritis in postpartum dairy cows. Theriogenology. https://doi.org/10.1016/j.theriogenology.2003.03.001.
Dubuc, J., Duffield, T. F., Leslie, K. E., Walton, J. S., & Leblanc, S. J. (2010). Definitions and diagnosis of postpartum endometritis in dairy cows. Journal of Dairy Science. https://doi.org/10.3168/jds.2010-3428.
Madoz, L. V., Giuliodori, M. J., Migliorisi, A. L., Jaureguiberry, M., & De Sota, R. L. (2014). Endometrial cytology , biopsy , and bacteriology for the diagnosis of subclinical endometritis in grazing dairy cows. Journal of Dairy Science. https://doi.org/10.3168/jds.2013-6836.
Gilbert, R. O., Shin, S. T., Guard, C. L., Erb, H. N., & Frajblat, M. (2005). Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology. https://doi.org/10.1016/j.theriogenology.2005.04.022.
Leblanc, S. J., Duffield, T. F., Leslie, K. E., Bateman, K. G., Keefe, G. P., Walton, J. S., & Johnson, W. H. (2002). Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J. Dairy Sci. https://doi.org/10.3168/jds.S0022-0302(02)74302-6.
Carneiro, L. C., Ferreira, A. F., Padua, M., Saut, J. P., Ferraudo, A. S., & dos Santos, R. M. (2014). Incidence of subclinical endometritis and its effects on reproductive performance of crossbred dairy cows. Tropical Animal Health and Production., 46(8), 1435–1439. https://doi.org/10.1007/s11250-014-0661-y.
Schaefer, T. M., Desouza, K., Fahey, J. V., Beagley, K. W., & Wira, C. R. (2004). Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology. https://doi.org/10.1111/j.1365-2567.2004.01898.x.
Herath, S., Fischer, D. P., Werling, D., Williams, E. J., Lilly, S. T., Dobson, H., et al. (2006). Expression and function of toll-like receptor 4 in the endometrial cells of the uterus. Endocrinology. https://doi.org/10.1210/en.2005-1113.
Reddy, S. P., Tran, K., Malik, A. B., Siddiqui, M. R., & Mittal, M. (2013). Reactive oxygen species in inflammation and tissue injury. Antioxidants & Redox Signaling. https://doi.org/10.1089/ars.2012.5149.
Mittal, M., Siddiqui, M., Tran, K., Reddy, S., & Malik, A. (2014). Reactive oxygen species in inflammation and tissue injury. Antioxidants & Redox Signaling. https://doi.org/10.1371/journal.pbio.1000479.
Nita, M., & Grzybowski, A. (2016). The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxidative Medicine and Cellular Longevity. https://doi.org/10.1155/2016/3164734.
Gaschler, M. M., & Stockwell, B. R. (2017). Lipid peroxidation in cell death. Biochemical and Biophysical Research Communications. https://doi.org/10.1016/j.bbrc.2016.10.086.
Bandyopadhyay, U., Das, D., & Banerjee, R. K. (1999). Reactive oxygen species: oxidative damage and pathogenesis. Current Science.
Pavlov, V. A., & Tracey, K. J. (2006). Controlling inflammation: the cholinergic anti-inflammatory pathway. Biochemical Society Transactions. https://doi.org/10.1042/bst0341037.
Tracey, K. J. (2009). Reflex control of immunity. Nature Reviews Immunology. https://doi.org/10.1038/nri2566.
Wang, H., Yu, M., Ochani, M., Amelia, C. A., Tanovic, M., Susarla, S., et al. (2003). Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. https://doi.org/10.1038/nature01339.
Rosas-Ballina, M., & Tracey, K. J. (2009). Cholinergic control of inflammation. In Journal of Internal Medicine. https://doi.org/10.1111/j.1365-2796.2009.02098.x.
Czura, C. J., & Tracey, K. J. (2013). The cholinergic anti-inflammatory pathway. In Autonomic Neuroimmunology. https://doi.org/10.3109/9780203008966.
Martelli, D., McKinley, M. J., & McAllen, R. M. (2014). The cholinergic anti-inflammatory pathway: a critical review. Autonomic Neuroscience: Basic and Clinical. https://doi.org/10.1016/j.autneu.2013.12.007.
Gallowitsch-Puerta, M., & Pavlov, V. A. (2007). Neuro-immune interactions via the cholinergic anti-inflammatory pathway. Life Sciences. https://doi.org/10.1016/j.lfs.2007.01.002.
Kawashima, K., & Fujii, T. (2003). The lymphocytic cholinergic system and its contribution to the regulation of immune activity. Life Sciences. https://doi.org/10.1016/j.lfs.2003.09.037.
Pavlov, V. A., & Tracey, K. J. (2004). Neural regulators of innate immune responses and inflammation. Cellular and Molecular Life Sciences. https://doi.org/10.1007/s00018-004-4102-3.
Taylor, P. (1991). The cholinesterases. Journal of Biological Chemistry.
Das, U. N. (2007). Acetylcholinesterase and butyrylcholinesterase as markers of low-grade systemic inflammation. Annals of Hepatology.
Blusztajn, J. K., & Wurtman, R. J. (1983). Choline and cholinergic neurons. Science. https://doi.org/10.1126/science.6867732.
Patocka, J., Kuca, K., & Jun, D. (2004). Acetylcholinesterase and butyrylcholinesterase—important enzymes of human body. Acta medica (Hradec Kralove).
Borovikova, L. V., Ivanova, S., Zhang, M., Yang, H., Botchkina, G. I., Watkins, L. R., et al. (2000). Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. https://doi.org/10.1038/35013070.
Tracey, K. J., Borovikova, L. V., Ivanova, S., Zhang, M., Yang, H., Botchkina, G. I., et al. (2000). Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. https://doi.org/10.1038/35013070.
de Jonge, W. J., van der Zanden, E. P., The, F. O., Bijlsma, M. F., van Westerloo, D. J., Bennink, R. J., et al. (2005). Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nature Immunology. https://doi.org/10.1038/ni1229.
Ramírez, M. J., Cenarruzabeitia, E., Lasheras, B., & Del Rio, J. (1997). 5-HT2receptor regulation of acetylcholine release induced by dopaminergic stimulation in rat striatal slices. Brain Research. https://doi.org/10.1016/S0006-8993(96)01434-5.
Arne Boyum. (1968). Isolation of mononuclear cells and granulocytes from human blood. Scand. J. C/in. Lab. Invest.
Jain, N. C. (Nemi C. (1993). Essentials of veterinary hematology. Published in 1993 in Philadelphia (Pa.) by Lea and Febiger.
Ellman, L. G., Courtney, K. D., Andres, V., & Featherstone, M. R. (1961). A new and rapid colorimetric of acetylcholinesterase determination. Biochemical Pharmacology.
Worek, F., Mast, U., Kiderlen, D., Diepold, C., & Eyer, P. (1999). Improved determination of acetylcholinesterase activity in human whole blood. Clinica Chimica Acta. https://doi.org/10.1016/S0009-8981(99)00144-8.
Fitzgerald, B. B., & Costa, L. G. (1993). Modulation of muscarinic receptors and acetylcholinesterase activity in lymphocytes and in brain areas following repeated organophosphate exposure in rats. Toxicological Sciences. https://doi.org/10.1093/toxsci/20.2.210.
Jentzsch, A. M., Bachmann, H., Fürst, P., & Biesalski, H. K. (1996). Improved analysis of malondialdehyde in human body fluids. Free Radical Biology and Medicine. https://doi.org/10.1016/0891-5849(95)02043-8.
Nelson, D. P., & Kiesow, L. A. (1972). Enthalpy of decomposition of hydrogen peroxide by catalase at 25?? C (with molar extinction coefficients of H2O2 solutions in the UV). Analytical Biochemistry, 49(2), 474–478. https://doi.org/10.1016/0003-2697(72)90451-4.
KIM, I.-H., NA, K.-J., & YANG, M.-P. (2006). Immune responses during the peripartum period in dairy cows with postpartum endometritis. Journal of Reproduction and Development. https://doi.org/10.1262/jrd.17036.
Subandrio, A. L., Sheldon, I. M., & Noakes, D. E. (2000). Peripheral and intrauterine neutrophil function in the cow: the influence of endogenous and exogenous sex steroid hormones. Theriogenology. https://doi.org/10.1016/S0093-691X(00)00300-9.
Subandrio, A., & Noakes, D. (1997). Neutrophil migration into the uterine lumen of the cow: the influence of endogenous and exogenous sex steroid hormones using two intrauterine chemoattractants. Theriogenology. https://doi.org/10.1016/S0093-691X(97)00038-1.
Castro, V. S. P., Da Silva, A. S., Costa, M. M., Paim, F. C., Alves, S. H., Lopes, S. T. A., et al. (2016). Cholinergic enzymes and inflammatory markers in rats infected by Sporothrix schenckii. Microbial Pathogenesis, 97. https://doi.org/10.1016/j.micpath.2016.05.020.
Kim, I. H., Kang, H. G., Jeong, J. K., Hur, T. Y., & Jung, Y. H. (2014). Inflammatory cytokine concentrations in uterine flush and serum samples from dairy cows with clinical or subclinical endometritis. Theriogenology. https://doi.org/10.1016/j.theriogenology.2014.04.022.
Galvão, K. N., Santos, N. R., Galvão, J. S., & Gilbert, R. O. (2011). Association between endometritis and endometrial cytokine expression in postpartum Holstein cows. Theriogenology. https://doi.org/10.1016/j.theriogenology.2011.02.006.
Cybulsky, M. I., Colditz, I. G., & Movat, H. Z. (1986). The role of interleukin-1 in neutrophil leukocyte emigration induced by endotoxin. Am J Pathol.
Tracey, K. J. (2002). The inflammatory reflex. Nature. https://doi.org/10.1038/nature01321.
Wang, H., Liao, H., Ochani, M., Justiniani, M., Lin, X., Yang, L., et al. (2004). Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nature Medicine. https://doi.org/10.1038/nm1124.
Ulloa, L. U. I. S. (2010). Cholinergic regulation of NF-kB in sepsis. RePORTER Database National Institutes of Health.
Li, B., Stribley, J. A., Ticu, A., Xie, W., Schopfer, L. M., Hammond, P., … Lockridge, O. (2000). Abundant tissue butyrylcholinesterase and its possible function in the acetylcholinesterase knockout mouse. Journal of neurochemistry.
Ben Assayag, E., Shenhar-Tsarfaty, S., Ofek, K., Soreq, L., Bova, I., Shopin, L., et al. (2010). Serum cholinesterase activities distinguish between stroke patients and controls and predict 12-month mortality. Molecular medicine (Cambridge, Mass.). https://doi.org/10.2119/molmed.2010.00015.
Tayebati, S. K., El-Assouad, D., Ricci, A., & Amenta, F. (2002). Immunochemical and immunocytochemical characterization of cholinergic markers in human peripheral blood lymphocytes. Journal of Neuroimmunology. https://doi.org/10.1016/S0165-5728(02)00325-9.
Kawashima, K., & Fujii, T. (2004). Expression of non-neuronal acetylcholine in lymphocytes and its contribution to the regulation of immune function. Frontiers in Bioscience. https://doi.org/10.2741/1390.
Fujii, T., Mashimo, M., Moriwaki, Y., Misawa, H., Ono, S., Horiguchi, K., & Kawashima, K. (2017). Expression and function of the cholinergic system in immune cells. Frontiers in Immunology. https://doi.org/10.3389/fimmu.2017.01085.
Kawashima, K., & Fujii, T. (2000). Extraneuronal cholinergic system in lymphocytes. Pharmacology and Therapeutics. https://doi.org/10.1016/S0163-7258(99)00071-6.
Hammon, D. S., Evjen, I. M., Dhiman, T. R., Goff, J. P., & Walters, J. L. (2006). Neutrophil function and energy status in Holstein cows with uterine health disorders. Veterinary Immunology and Immunopathology. https://doi.org/10.1016/j.vetimm.2006.03.022.
Sheldon, I. M., & Dobson, H. (2004). Postpartum uterine health in cattle. In Animal Reproduction Science. https://doi.org/10.1016/j.anireprosci.2004.04.006.
Teng, T.-S., Ji, A., Ji, X.-Y., & Li, Y.-Z. (2017). Neutrophils and immunity: from bactericidal action to being conquered. Journal of Immunology Research. https://doi.org/10.1155/2017/9671604.
LeBlanc, S. J., Osawa, T., & Dubuc, J. (2011). Reproductive tract defense and disease in postpartum dairy cows. Theriogenology. https://doi.org/10.1016/j.theriogenology.2011.07.017.
Parrilla-Hernandez, S., Ponthier, J. Ô., Franck, T. Y., Serteyn, D. D., & Deleuze, S. C. (2014). High concentrations of myeloperoxidase in the equine uterus as an indicator of endometritis. Theriogenology. https://doi.org/10.1016/j.theriogenology.2014.01.011.
El-Benna, J., Dang, P. M. C., Gougerot-Pocidalo, M. A., Marie, J. C., & Braut-Boucher, F. (2009). p47phox, the phagocyte NADPH oxidase/NOX2 organizer: Structure, phosphorylation and implication in diseases. Experimental and Molecular Medicine. https://doi.org/10.3858/emm.2009.41.4.058.
Lykkesfeldt, J., & Svendsen, O. (2007). Oxidants and antioxidants in disease: Oxidative stress in farm animals. Veterinary Journal. https://doi.org/10.1016/j.tvjl.2006.06.005.
Chihuailaf, R. H., Contreras, P. A., & Wittwer, F. G. (2002). Patogénesis del estrés oxidativo : consecuencias y evaluación en salud animal. Veterinaria México.
Yu, B. P. (2017). Cellular defenses against damage from reactive oxygen species. Physiological Reviews. https://doi.org/10.1152/physrev.1994.74.1.139.
Martinez, G. R., Loureiro, A. P. M., Marques, S. A., Miyamoto, S., Yamaguchi, L. F., Onuki, J., et al. (2003). Oxidative and alkylating damage in DNA. In Mutation Research - Reviews in Mutation Research. https://doi.org/10.1016/j.mrrev.2003.05.005.
Circu, M. L., & Aw, T. Y. (2010). Reactive oxygen species, cellular redox systems, and apoptosis. Free Radical Biology and Medicine. https://doi.org/10.1016/j.freeradbiomed.2009.12.022.
Ighodaro, O. M., & Akinloye, O. A. (2017). First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine. https://doi.org/10.1016/j.ajme.2017.09.001.
Acknowledgments
We are thankful to the Secretary of Economic Development, Science and Technology of the state of Rio Grande do Sul (SDECT-RS) and the Center of Technological Innovation of Alto Jacuí (Inovatec).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state that they have no conflict of interest to declare. The procedure was approved by the Animal Welfare Committee of UNICRUZ, number 07/2017, in accordance to Brazilian laws and ethical principles published by the Colégio Brasileiro de Experimentação Animal (COBEA).
All authors agreed with the subscription of the article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Siqueira, L.C., Favaretto, B., Moraes, B.T. et al. Bovine Endometritis and the Inflammatory Peripheral Cholinergic System. Appl Biochem Biotechnol 190, 1242–1256 (2020). https://doi.org/10.1007/s12010-019-03157-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03157-0