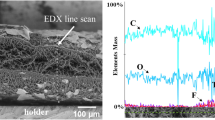
Abstract
Tissue engineering makes it possible to fabricate scaffolds that can help the function of defective tissues or even the most complex organs such as the heart. Carbon nanofibers (CNFs), because of their high mechanical strength and electrical properties, can improve the functional coupling of cardiomyocytes and their electrophysiological properties. In this study, electroactive CNF/gelatin (Gel) nanofibrous cardiac patches were prepared by an electrospinning method. Scanning electron microscope (SEM) evaluation of prepared scaffolds showed randomly oriented nanofibers. The electrical conductivity of the CNF/Gel scaffolds was assessed by a four-probe device and was in the semiconducting range (~ 10−5 S/m). The result of an MTT assay confirmed the excellent biocompatibility of electroactive CNF/Gel scaffolds. Also, CNF-containing scaffolds supported cardiomyocyte adhesion and increased expression of the cardiac genes including TrpT-2, Actn4, and Conx43 compared with the non-conductive counterpart. Our findings also confirmed the angiogenic potential of CNF/Gel scaffolds as compatible and electroactive platforms for cardiac tissue engineering.









Similar content being viewed by others
References
Schürlein, S., Al Hijailan, R., Weigel, T., Kadari, A., Rücker, C., Edenhofer, F., Walles, H., & Hansmann, J. (2017). Generation of a human cardiac patch based on a reendothelialized biological scaffold (BioVaSc). Advanced Biosystems, 1.
Heusch, G., & Gersh, B. J. (2016). The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. European Heart Journal, 38, 774–784.
Wang, Q., Yang, H., Bai, A., Jiang, W., Li, X., Wang, X., Mao, Y., Lu, C., Qian, R., & Guo, F. (2016). Functional engineered human cardiac patches prepared from nature’s platform improve heart function after acute myocardial infarction. Biomaterials, 105, 52–65.
Prabhakaran, M. P., Venugopal, J., Kai, D., & Ramakrishna, S. (2011). Biomimetic material strategies for cardiac tissue engineering. Materials Science and Engineering: C, 31(3), 503–513.
Weinberger, F., Mannhardt, I., & Eschenhagen, T. (2017). Engineering cardiac muscle tissue: a maturating field of research. Circulation Research, 120(9), 1487–1500.
Venugopal, J. R., Prabhakaran, M. P., Mukherjee, S., Ravichandran, R., Dan, K., & Ramakrishna, S. (2012). Biomaterial strategies for alleviation of myocardial infarction. Journal of the Royal Society Interface, 9(66), 1–19.
Saidi, R., & Kenari, S. H. (2014). Challenges of organ shortage for transplantation: solutions and opportunities. International Journal of Organ Transplantation Medicine, 5, 87.
Schaefer, J. A., Guzman, P. A., Riemenschneider, S. B., Kamp, T. J., & Tranquillo, R. T. (2018). A cardiac patch from aligned microvessel and cardiomyocyte patches. Journal of Tissue Engineering and Regenerative Medicine, 12(2), 546–556.
Baheiraei, N., Gharibi, R., Yeganeh, H., Miragoli, M., Salvarani, N., Di Pasquale, E., & Condorelli, G. (2016). Electroactive polyurethane/siloxane derived from castor oil as a versatile cardiac patch, part I: Synthesis, characterization, and myoblast proliferation and differentiation. Journal of Biomedical Materials Research Part A, 104(3), 775–787.
Prabhakaran, M. P., Kai, D., Ghasemi-Mobarakeh, L., & Ramakrishna, S. (2011). Electrospun biocomposite nanofibrous patch for cardiac tissue engineering. Biomedical Materials, 6(5), 055001.
Kitsara, M., Agbulut, O., Kontziampasis, D., Chen, Y., & Menasché, P. (2017). Fibers for hearts: a critical review on electrospinning for cardiac tissue engineering. Acta Biomaterialia, 48, 20–40.
Ketabchi, N., Naghibzadeh, M., Adabi, M., Esnaashari, S. S., & Faridi-Majidi, R. (2017). Preparation and optimization of chitosan/polyethylene oxide nanofiber diameter using artificial neural networks. Neural Computing and Applications, 28(11), 3131–3143.
Chow, L. W. (2018). Electrospinning functionalized polymers for use as tissue engineering scaffolds (pp. 27–39). Springer.
Dozois, M. D., Bahlmann, L. C., Zilberman, Y., & Tang, X. S. (2017). Carbon nanomaterial-enhanced scaffolds for the creation of cardiac tissue constructs: a new frontier in cardiac tissue engineering. Carbon, 120, 338–349.
Naghibzadeh, M., Firoozi, S., Nodoushan, F. S., Adabi, M., Khoradmehr, A., Fesahat, F., Esnaashari, S. S., Khosravani, M., Tavakol, S., & Pazoki-Toroudi, H. (2018). Application of electrospun gelatin nanofibers in tissue engineering. Biointerface Research in Applied Chemistry, 8, 3048–3052.
Gnavi, S., Blasio, L., Tonda-Turo, C., Mancardi, A., Primo, L., Ciardelli, G., Gambarotta, G., Geuna, S., & Perroteau, I. (2017). Gelatin-based hydrogel for vascular endothelial growth factor release in peripheral nerve tissue engineering. Journal of Tissue Engineering and Regenerative Medicine, 11(2), 459–470.
Echave, M. C., S Burgo, L., Pedraz, J. L., & Orive, G. (2017). Gelatin as biomaterial for tissue engineering. Current Pharmaceutical Design, 23, 3567–3584.
Chan, Y.-C., Ting, S., Lee, Y.-K., Ng, K.-M., Zhang, J., Chen, Z., Siu, C.-W., Oh, S. K., & Tse, H.-F. (2013). Electrical stimulation promotes maturation of cardiomyocytes derived from human embryonic stem cells. Journal of Cardiovascular Translational Research, 6(6), 989–999.
Tandon, N., Cannizzaro, C., Chao, P.-H. G., Maidhof, R., Marsano, A., Au, H. T. H., Radisic, M., & Vunjak-Novakovic, G. (2009). Electrical stimulation systems for cardiac tissue engineering. Nature Protocols, 4(2), 155–173.
Kankala, R. K., Zhu, K., Sun, X.-N., Liu, C.-G., Wang, S.-B., & Chen, A.-Z. (2018). Cardiac tissue engineering on the nanoscale. ACS Biomaterials Science & Engineering, 4(3), 800–818.
Agyemang, F. O., Tomboc, G. M., Kwofie, S., & Kim, H. (2017). Electrospun carbon nanofiber-carbon nanotubes coated polyaniline composites with improved electrochemical properties for supercapacitors. Electrochimica Acta.
Samadian, H., Zakariaee, S. S., Adabi, M., Mobasheri, H., Azami, M., & Faridi-Majidi, R. (2016). Effective parameters on conductivity of mineralized carbon nanofibers: an investigation using artificial neural networks. RSC Advances, 6(113), 111908–111918.
Chen, X., Wu, Y., Ranjan, V. D., & Zhang, Y. (2018). Three-dimensional electrical conductive scaffold from biomaterial-based carbon microfiber sponge with bioinspired coating for cell proliferation and differentiation. Carbon, 134, 174–182.
El-Aziz, A. A., El Backly, R. M., Taha, N. A., El-Maghraby, A., & Kandil, S. H. (2017). Preparation and characterization of carbon nanofibrous/hydroxyapatite sheets for bone tissue engineering. Materials Science and Engineering: C, 76, 1188–1195.
Stout, D. A., Basu, B., & Webster, T. J. (2011). Poly (lactic–co-glycolic acid): carbon nanofiber composites for myocardial tissue engineering applications. Acta Biomaterialia, 7(8), 3101–3112.
Martins, A. M., Eng, G., Caridade, S. G., Mano, J. O. F., Reis, R. L., & Vunjak-Novakovic, G. (2014). Electrically conductive chitosan/carbon scaffolds for cardiac tissue engineering. Biomacromolecules, 15(2), 635–643.
Adabi, M., Saber, R., Naghibzadeh, M., Faridbod, F., & Faridi-Majidi, R. (2015). Parameters affecting carbon nanofiber electrodes for measurement of cathodic current in electrochemical sensors: an investigation using artificial neural network. RSC Advances, 5(99), 81243–81252.
Adabi, M., Saber, R., Faridi-Majidi, R., & Faridbod, F. (2015). Performance of electrodes synthesized with polyacrylonitrile-based carbon nanofibers for application in electrochemical sensors and biosensors. Materials Science and Engineering: C, 48, 673–678.
Bidez, P. R., Li, S., MacDiarmid, A. G., Venancio, E. C., Wei, Y., & Lelkes, P. I. (2006). Polyaniline, an electroactive polymer, supports adhesion and proliferation of cardiac myoblasts. Journal of Biomaterials Science, Polymer Edition, 17(1-2), 199–212.
Baheiraei, N., Yeganeh, H., Ai, J., Gharibi, R., Azami, M., & Faghihi, F. (2014). Synthesis, characterization and antioxidant activity of a novel electroactive and biodegradable polyurethane for cardiac tissue engineering application. Materials Science and Engineering: C, 44, 24–37.
Baheiraei, N., Yeganeh, H., Ai, J., Gharibi, R., Ebrahimi-Barough, S., Azami, M., Vahdat, S., & Baharvand, H. (2015). Preparation of a porous conductive scaffold from aniline pentamer-modified polyurethane/PCL blend for cardiac tissue engineering. Journal of Biomedical Materials Research Part A, 103(10), 3179–3187.
Khorramirouz, R., Go, J. L., Noble, C., Jana, S., Maxson, E., Lerman, A., & Young, M. D. (2018). A novel surgical technique for a rat subcutaneous implantation of a tissue engineered scaffold. Acta Histochemica, 120(3), 282–291.
Jalise, S. Z., Baheiraei, N., & Bagheri, F. (2018). The effects of strontium incorporation on a novel gelatin/bioactive glass bone graft: In vitro and in vivo characterization. Ceramics International, 44(12), 14217–14227.
Cuesta, A., Dhamelincourt, P., Laureyns, J., Martinez-Alonso, A., & Tascon, J. M. (1998). Comparative performance of X-ray diffraction and Raman microprobe techniques for the study of carbon materials. Journal of Materials Chemistry, 8(12), 2875–2879.
Lee, J.-W., Serna, F., Nickels, J., & Schmidt, C. E. (2006). Carboxylic acid-functionalized conductive polypyrrole as a bioactive platform for cell adhesion. Biomacromolecules, 7(6), 1692–1695.
Dai, X., Huang, Y.-C., Leichner, J., Nair, M., Lin, W.-C., & Li, C.-Z. (2015). Peptide modified polymer poly (glycerol-dodecanedioate co-fumarate) for efficient control of motor neuron differentiation. Biomedical Materials, 10(6), 065013.
Kai, D., Prabhakaran, M. P., Jin, G., & Ramakrishna, S. (2011). Guided orientation of cardiomyocytes on electrospun aligned nanofibers for cardiac tissue engineering. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 98, 379–386.
Tresoldi, C., Peneda Pacheco, D. P., Formenti, E., Gentilini, R., Mantero, S., & Petrini, P. (2017). Alginate/gelatin hydrogels to coat porous tubular scaffolds for vascular tissue engineering. European Cells & Materials, 33.
Martinelli, V., Cellot, G., Toma, F. M., Long, C. S., Caldwell, J. H., Zentilin, L., Giacca, M., Turco, A., Prato, M., & Ballerini, L. (2013). Carbon nanotubes instruct physiological growth and functionally mature syncytia: nongenetic engineering of cardiac myocytes. ACS Nano, 7(7), 5746–5756.
Martinelli, V., Cellot, G., Toma, F. M., Long, C. S., Caldwell, J. H., Zentilin, L., Giacca, M., Turco, A., Prato, M., & Ballerini, L. (2012). Carbon nanotubes promote growth and spontaneous electrical activity in cultured cardiac myocytes. Nano Letters, 12(4), 1831–1838.
Kumar, R., Meyyappan, M., & Koehne, J. E. The electrochemical society (p. 2502).
Baker, S. R., Banerjee, S., Bonin, K., & Guthold, M. (2016). Determining the mechanical properties of electrospun poly-ε-caprolactone (PCL) nanofibers using AFM and a novel fiber anchoring technique. Materials Science and Engineering: C, 59, 203–212.
Omens, J. H. (1998). Stress and strain as regulators of myocardial growth. Progress in Biophysics and Molecular Biology, 69(2-3), 559–572.
Kotwal, A., & Schmidt, C. E. (2001). Electrical stimulation alters protein adsorption and nerve cell interactions with electrically conducting biomaterials. Biomaterials, 22(10), 1055–1064.
Zhang, X.-R., Hu, X.-Q., Jia, X.-L., Yang, L.-K., Meng, Q.-Y., Shi, Y.-Y., Zhang, Z.-Z., Cai, Q., Ao, Y.-F., & Yang, X.-P. (2016). Cell studies of hybridized carbon nanofibers containing bioactive glass nanoparticles using bone mesenchymal stromal cells. Scientific Reports, 6(1), 38685.
Monteiro, L. M., Vasques-Novoa, F., Ferreira, L., & Nascimento, D. S. (2017). Restoring heart function and electrical integrity: closing the circuit. NPJ Regenerative Medicine, 2(1), 9.
Kharaziha, M., Shin, S. R., Nikkhah, M., Topkaya, S. N., Masoumi, N., Annabi, N., Dokmeci, M. R., & Khademhosseini, A. (2014). Tough and flexible CNT–polymeric hybrid scaffolds for engineering cardiac constructs. Biomaterials, 35(26), 7346–7354.
Pok, S., Vitale, F., Eichmann, S. L., Benavides, O. M., Pasquali, M., & Jacot, J. G. (2014). Biocompatible carbon nanotube–chitosan scaffold matching the electrical conductivity of the heart. ACS Nano, 8(10), 9822–9832.
Shin, S. R., Jung, S. M., Zalabany, M., Kim, K., Zorlutuna, P., Kim, S. B., Nikkhah, M., Khabiry, M., Azize, M., & Kong, J. (2013). Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano, 7(3), 2369–2380.
Sun, H., Lü, S., Jiang, X.-X., Li, X., Li, H., Lin, Q., Mou, Y., Zhao, Y., Han, Y., & Zhou, J. (2015). Carbon nanotubes enhance intercalated disc assembly in cardiac myocytes via the β1-integrin-mediated signaling pathway. Biomaterials, 55, 84–95.
Zhou, J., Chen, J., Sun, H., Qiu, X., Mou, Y., Liu, Z., Zhao, Y., Li, X., Han, Y., & Duan, C. (2014). Engineering the heart: evaluation of conductive nanomaterials for improving implant integration and cardiac function. Scientific Reports, 4, 3733.
Naskar, D., Ghosh, A. K., Mandal, M., Das, P., Nandi, S. K., & Kundu, S. C. (2017). Dual growth factor loaded nonmulberry silk fibroin/carbon nanofiber composite 3D scaffolds for in vitro and in vivo bone regeneration. Biomaterials, 136, 67–85.
Blazewicz, M. (2001). Carbon materials in the treatment of soft and hard tissue injuries. European Cells & Materials, 2, 21–29.
Jell, G., Verdejo, R., Safinia, L., Shaffer, M. S., Stevens, M. M., & Bismarck, A. (2008). Carbon nanotube-enhanced polyurethane scaffolds fabricated by thermally induced phase separation. Journal of Materials Chemistry, 18(16), 1865–1872.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Research Involving Human Participants and/or Animals
Animal experiments were performed according to the ethics committee guidelines for laboratory animals approved by the Ethics Committee of Tarbiat Modares University, Iran (IR.TMU.REC.1395.440).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mehrabi, A., Baheiraei, N., Adabi, M. et al. Development of a Novel Electroactive Cardiac Patch Based on Carbon Nanofibers and Gelatin Encouraging Vascularization. Appl Biochem Biotechnol 190, 931–948 (2020). https://doi.org/10.1007/s12010-019-03135-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03135-6