Abstract
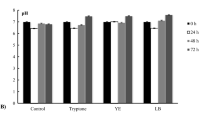
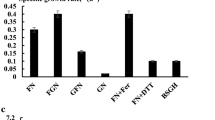
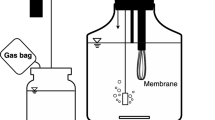
To recover a nitrogen resource from high-ammonia-nitrogen wastewater, two amphitrophic hydrogen-oxidizing bacteria (HOB), Paracoccus denitrificans Y5 and P. versutus D6, capable of nitrogen assimilation for single-cell protein (SCP) production were isolated. These two HOB strains could grow autotrophically with H2 as an electron donor, O2 as an electron acceptor, CO2 as a carbon source, and ammonia nitrogen (NH4+-N) as a nitrogen source. The cell molecular formulas of strains Y5 and D6 determined by autotrophic cultivation were C3.33H6.83O2.58N0.77 and C2.87H5.34O3.17N0.57, respectively. The isolated strains could synchronously remove NH4+-N and organic carbon and produce SCP via heterotrophic cultivation. The rates of removal of NH4+-N and soluble chemical oxygen demand reached 35.47 and 49.04%, respectively, for Y5 under mixotrophic cultivation conditions with biogas slurry as a substrate. SCP content of strains Y5 and D6 was 67.34–73.73% based on cell dry weight. Compared with soybean meal, the SCP of Y5 contained a variety of amino acids.






Similar content being viewed by others
References
Xing, W., Zhang, W. Q., Li, D. S., Li, J. L., Jia, F. F., Cui, Y. W., & Ren, F. M. (2017). An integrated O/A two-stage packed-bed reactor (INT-PBR) for total nitrogen removal from low organic carbon wastewater. Chemical Engineering Journal, 328, 894–903.
Khardenavis, A. A., Kapley, A., & Purohit, H. J. (2007). Simultaneous nitrification and denitrification by diverse Diaphorobacter sp. Applied Microbial and Cell Physiology, 77, 403–409.
Medhi, K., Singhal, A., Chauhan, D. K., & Thakur, I. S. (2017). Investigating the nitrification and denitrification kinetics under aerobic and anaerobic conditions by Paracoccus denitrificans ISTOD1. Bioresource Technology, 242, 334–343.
Hou, J., Xia, L., Ma, T., Zhang, Y. Q., Zhou, Y. Y., & He, X. G. (2017). Achieving short-cut nitrification and denitrification in modified intermittently aerated constructed wetland. Bioresource Technology, 232, 10–17.
Wei, H. W., Wang, J., Hassan, M., Han, L., & Xie, B. (2017). Anaerobic ammonium oxidation-denitrification synergistic interaction of mature landfill leachate in aged refuse bioreactor: Variations and effects of microbial community structures. Bioresource Technology, 243, 1149–1158.
Jalasutram, V., Kataram, S., Gandu, B., & Anupoju, G. (2013). Single cell protein production from digested and undigested poultry litter by Candida utilis: optimization of process parameters using response surface methodology. Clean Technologies and Environmental Policy, 15(2), 265–273.
Matassa, S., Boon, N., Arends, J. B. A., & Verstraete, W. (2015). H2-oxidizing bacteria for single cell protein production and sustainable nitrogen cycling, Resource Recovery, 1st IWA Conference, Abstracts.
Nangul, A., & Bhatia, R. (2013). Microorganisms: a marvelous source of single cell proteins. The Journal of Microbiology, Biotechnology and Food Sciences, 31, 15–18.
Nasseri, A. T., Rasoul-Amini, S., Morowvat, M. H., & Ghasemi, Y. (2011). Single cell protein: production and process. American Journal of Food Technology, 6, 103–116.
Pohlmann, A., Fricke, W. F., Reinecke, F., Kusian, B., Liesegang, H., Cramm, R., Eitinger, T., Ewering, C., Potter, M., Schwartz, E., Strittmatter, A., Vosz, I., Gottschalk, G., Steinbuchel, A., Friedrich, B., & Bowien, B. (2006). Genome sequence of the bioplastic-producing “Knallgas” bacterium Ralstonia eutropha H16. Nature Biotechnology, 24(10), 1257–1262.
Matassa, S., Boon, N., & Verstraete, W. (2015). Resource recovery from used water: the manufacturing abilities of hydrogen-oxidizing bacteria. Water Research, 68, 467–478.
Anupama, & Ravindra, P. (2000). Value-added food: single cell protein. Biotechnology Advances, 18(6), 459–479.
Repaske, R. (1966). Characteristics of hydrogen bacteria. Biotechnology and Bioengineering, 8(2), 217–235.
Volova, T. G., & Barashkov, V. A. (2010). Characteristics of proteins synthesized by hydrogen-oxidizing microorganisms. Applied Biochemistry and Microbiology, 46(6), 574–579.
Aragno, M., & Schlegel, H. G. (1978). Physiological characterization of the hydrogen bacterium Aquaspirillum autotrophicum. Archives of Microbiology, 116(3), 221–229.
Vandamme, P., & Coenye, T. (2004). Taxonomy of the genus Cupriavidus: a tale of lost and found. International Journal of Systematic and Evolutionary Microbiology, 54(6), 2285–2289.
Yu, J., Dow, A., & Pingali, S. (2013). The energy efficiency of carbon dioxide fixation by a hydrogen-oxidizing bacterium. International Journal of Hydrogen Energy, 38(21), 8683–8690.
Hu, J., Wang, L., Li, Y., Fu, X., Le, Y., Xu, D., Lu, B., & Yu, J. (2009). Breeding, optimization and community structure analysis of non-photosynthetic CO2 assimilation microbial flora. Environmental Science, 30, 2438–2444.
Bae, S., Kwak, K., Kim, S., Chung, S. Y., & Igarashi, Y. (2001). Isolation and characterization of CO2-fixing hydrogen-oxidizing marine bacteria. Journal of Bioscience and Bioengineering, 91(5), 442–448.
Nguyen, S., Ala, F., Cardwell, C., Cai, D., McKindles, K. M., Lotvola, A., Hodges, S., Deng, Y., & Tiquia-Arashiro, S. M. (2013). Isolation and screening of carboxydotrophs isolated from composts and their potential for butanol synthesis. Environmental Technology, 34(13-14), 1995–2007.
APHA. (1998). Standard methods for the examination of water and wastewater. 21st Edition. American Public Health Association.
Tanaka, K., Miyawaki, K., Yamaguchi, A., Khosravi-Darani, K., & Matsusaki, H. (2011). Cell growth and P(3HB) accumulation from CO2 of a carbon monoxide-tolerant hydrogen-oxidizing bacterium, Ideonella sp. O-1. Applied Microbiology and Biotechnology, 92(6), 1161–1169.
Garcia-Gonzalez, L., Mozumder, M. S. I., Dubreuil, M., Volcke, E. I. P., & De Wever, H. (2015). Sustainable autotrophic production of polyhydroxybutyrate (PHB) from CO2 using a two-stage cultivation system. Catalysis Today, 257, 237–245.
Repaske, R. (1962). Nutritional requirements for Hydrogenomonas eutropha. Journal of Bacteriology, 83, 418–422.
Cohen, J. S., & Burris, R. H. (1955). A method for the culture of hydrogen bacteria. Journal of Bacteriology, 69(3), 316–319.
Matassa, S., Verstraete, W., Pikaar, I., & Boon, N. (2016). Autotrophic nitrogen assimilation and carbon capture for microbial protein production by a novel enrichment of hydrogen-oxidizing bacteria. Water Research, 101, 137–146.
Lo, S. N., & Moreau, J. R. (1986). Mixed-culture microbial protein from waste sulfite pulping liquor II: its production on pilot-plant scale and use in animal feed. Canadian Journal of Chemical Engineering, 64(4), 639–646.
Acknowledgements
This study was supported jointly by the National Key R & D Program of China (2018YFD0501405), by the Youth Innovation Promotion Association CAS (2017423), the Key Project for Foreign Cooperation of the International Cooperation Bureau of the Chinese Academy of Sciences (182344KYSB20170009), the Science and Technology Service Network Initiative (STS) of the Chinese Academy of Sciences, the Key Laboratory of Environmental and Applied Microbiology of Chengdu Institute of Biology CAS (KLCAS-2016-10, KLCAS-2017-9), and the Chengdu Science and Technology Huimin Project (2016-HM02-00092-SF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Dou, J., Huang, Y., Ren, H. et al. Autotrophic, Heterotrophic, and Mixotrophic Nitrogen Assimilation for Single-Cell Protein Production by Two Hydrogen-Oxidizing Bacterial Strains. Appl Biochem Biotechnol 187, 338–351 (2019). https://doi.org/10.1007/s12010-018-2824-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-018-2824-1