Abstract
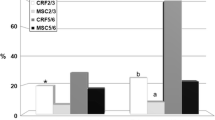
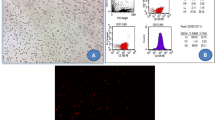
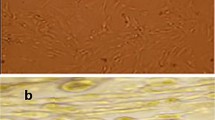
Chronic kidney disease may lead to subsequent tissue fibrosis. However, many factors can combat injurious stimuli in these tissues aiming to repair, heal, and alleviate any disturbance. Chemokines release, migration of inflammatory cells to the affected site, and activation of fibroblasts for the production of extracellular matrix are commonly observed in this disease. In the last years, many studies have focused on spironolactone (SPL), a mineralocorticoid receptor antagonist, and its pharmacological effects. In the present study, SPL was selected as an anti-inflammatory agent to combat nephrotoxicity and renal fibrosis induced by cisplatin. Mesenchymal stem cells (MSCs) were also selected in addition as a referring agent. Renal fibrosis induced by cisplatin intake significantly increased creatinine, urea, nuclear factor kappa B, insulin-like growth factor-1, fibroblast growth factor-23, and kidney malondialdehyde (MDA) content. Hepatocyte growth factor and renal content of reduced glutathione demonstrated a significant decrease. Histopathological examination of kidney tissues demonstrated marked cellular changes which are correlated with the biochemical results. Oral SPL intake (20 mg/kg/body weight) daily for 4 weeks and MSCs administration (3 × 106 cell/rat) intravenous to the experimental rats resulted in a significant improvement of both the biomarkers studied and the histopathological profile of the renal tissue. Individual administration of spironolactone and MSCs exhibited a marked anti-inflammatory potential and alleviated to a great extent the nephrotoxicity and renal fibrotic pattern induced by cisplatin.



Similar content being viewed by others
References
Cepeda, V., Fuertes, M. A., Castilla, J., Alonso, C., Quevedo, C., & Perez, J. M. (2007). Biochemical mechanisms of cisplatin cytotoxicity. Anti-Cancer Agents in Medicinal Chemistry, 7(1), 3–18.
Sancho-Martinez, S. M., Prieto-Garcia, L., Prieto, M., Lopez-Novoa, J. M., & Lopez-Hernandez, F. J. (2012). Subcellular targets of cisplatin cytotoxicity: an integrated view. Pharmacology & Therapeutics, 136(1), 35–55.
Zhao, J.-l., Zhao, J., & Jiao, H.-j. (2014). Synergistic growth-suppressive effects of quercetin and cisplatin on HepG2 human hepatocellular carcinoma cells. Applied Biochemistry and Biotechnology, 172(2), 784–791.
Liu, Y. (2004). Hepatocyte growth factor in kidney fibrosis: therapeutic potential and mechanisms of action. American journal of physiology Renal Physiology, 287(1), F7–16.
Eddy, A. A. (2000). Molecular basis of renal fibrosis. Pediatric Nephrology, 15(3–4), 290–301.
Klahr, S., & Morrissey, J. (2002). Obstructive nephropathy and renal fibrosis. American Journal of Physiology Renal Physiology, 283(5), F861–F875.
Eddy, A. A. (1996). Molecular insights into renal interstitial fibrosis. Journal of the American Society of Nephrology : JASN, 7(12), 2495–2508.
Strutz, F., Zeisberg, M., Hemmerlein, B., Sattler, B., Hummel, K., Becker, V., & Muller, G. A. (2000). Basic fibroblast growth factor expression is increased in human renal fibrogenesis and may mediate autocrine fibroblast proliferation. Kidney International, 57(4), 1521–1538.
el Nahas, A. M., Muchaneta-Kubara, E. C., Essawy, M., & Soylemezoglu, O. (1997). Renal fibrosis: insights into pathogenesis and treatment. The International Journal of Biochemistry & Cell Biology, 29(1), 55–62.
Boor, P., Ostendorf, T., & Floege, J. (2010). Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nature Reviews. Nephrology, 6(11), 643–656.
Decleves, A. E., & Sharma, K. (2014). Novel targets of antifibrotic and anti-inflammatory treatment in CKD. Nature Reviews. Nephrology, 10(5), 257–267.
Ziyadeh, F. N., Hoffman, B. B., Han, D. C., Iglesias-De La Cruz, M. C., Hong, S. W., Isono, M., Chen, S., McGowan, T. A., & Sharma, K. (2000). Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proceedings of the National Academy of Sciences of the United States of America, 97(14), 8015–8020.
Chen, S., Iglesias-de la Cruz, M. C., Jim, B., Hong, S. W., Isono, M., & Ziyadeh, F. N. (2003). Reversibility of established diabetic glomerulopathy by anti-TGF-beta antibodies in db/db mice. Biochemical and Biophysical Research Communications, 300(1), 16–22.
Guan, Q., Li, S., Gao, S., Chen, H., Nguan, C. Y., & Du, C. (2013). Reduction of chronic rejection of renal allografts by anti-transforming growth factor-beta antibody therapy in a rat model. American journal of physiology Renal Physiology, 305(2), F199–F207.
Williams, S. J., Zammit, S. C., Cox, A. J., Shackleford, D. M., Morizzi, J., Zhang, Y., Powell, A. K., Gilbert, R. E., Krum, H., & Kelly, D. J. (2013). 3′,4′-Bis-difluoromethoxycinnamoylanthranilate (FT061): an orally-active antifibrotic agent that reduces albuminuria in a rat model of progressive diabetic nephropathy. Bioorganic & Medicinal Chemistry Letters, 23(24), 6868–6873.
Sharma, K., McCue, P., & Dunn, S. R. (2003). Diabetic kidney disease in the db/db mouse. American journal of physiology Renal Physiology, 284(6), F1138–F1144.
Negri, A. L. (2004). Prevention of progressive fibrosis in chronic renal diseases: antifibrotic agents. Journal of Nephrology, 17(4), 496–503.
Zeisberg, M., & Kalluri, R. (2008). Reversal of experimental renal fibrosis by BMP-7 provides insights into novel therapeutic strategies for chronic kidney disease. Pediatric Nephrology, 23(9), 1395–1398.
Lippincott's Illustrated Pharmacology Review. 1997: p. 232.
Cai, C., L. Hou, J. Zhang, D. Zhao, Z. Wang, H. Hu, J. He, W. Guan, and Y. Ma, (2017) The inhibitory effect of mesenchymal stem cells with rAd-NK4 on liver cancer. Applied Biochemistry and Biotechnology, p. 1–16.
Wu, H. T., Sie, S. S., Kuan, T. C., & Lin, C. S. (2013). Identifying the regulative role of NF-κB binding sites within promoter region of human matrix metalloproteinase 9 (mmp-9) by TNF-α induction. Applied Biochemistry and Biotechnology, 169(2), 438–449.
Hassan, H. A., & El-Gharib, N. E. (2015). Obesity and clinical riskiness relationship: therapeutic management by dietary antioxidant supplementation—a review. Applied Biochemistry and Biotechnology, 176(3), 647–669.
Chen, X., & Xu, C. (2017). Proteomic analysis reveals the contribution of TGFβ/Smad4 signaling pathway to cell differentiation during planarian tail regeneration. Applied Biochemistry and Biotechnology, 182(2), 529–545.
Imberti, B., Morigi, M., Tomasoni, S., Rota, C., Corna, D., Longaretti, L., Rottoli, D., Valsecchi, F., Benigni, A., & Wang, J. (2007). Insulin-like growth factor-1 sustains stem cell-mediated renal repair. Journal of the American Society of Nephrology, 18(11), 2921–2928.
Eliopoulos, N., Zhao, J., Bouchentouf, M., Forner, K., Birman, E., Yuan, S., Boivin, M.-N., & Martineau, D. (2010). Human marrow-derived mesenchymal stromal cells decrease cisplatin renotoxicity in vitro and in vivo and enhance survival of mice post-intraperitoneal injection. American Journal of Physiology-Renal Physiology, 299(6), F1288–F1298.
Lee, R. H., Seo, M. J., Reger, R. L., Spees, J. L., Pulin, A. A., Olson, S. D., & Prockop, D. J. (2006). Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proceedings of the National Academy of Sciences, 103(46), 17438–17443.
Ezquer, F. E., Ezquer, M. E., Parrau, D. B., Carpio, D., Yañez, A. J., & Conget, P. A. (2008). Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biology of Blood and Marrow Transplantation, 14(6), 631–640.
Ezquer, F., Ezquer, M., Simon, V., Pardo, F., Yañez, A., Carpio, D., & Conget, P. (2009). Endovenous administration of bone marrow-derived multipotent mesenchymal stromal cells prevents renal failure in diabetic mice. Biology of Blood and Marrow Transplantation, 15(11), 1354–1365.
Alhadlaq, A., & Mao, J. J. (2004). Mesenchymal stem cells: isolation and therapeutics. Stem Cells and Development, 13(4), 436–448.
Jiang, P., Dong, Z., Ma, B., Ni, Z., Duan, H., Li, X., Wang, B., Ma, X., Wei, Q., & Ji, X. (2016). Effect of vanadyl rosiglitazone, a new insulin-mimetic vanadium complexes, on glucose homeostasis of diabetic mice. Applied Biochemistry and Biotechnology, 180(5), 841–851.
Brilla, C. G., Matsubara, L. S., & Weber, K. T. (1993). Antifibrotic effects of spironolactone in preventing myocardial fibrosis in systemic arterial hypertension. The American Journal of Cardiology, 71(3), A12–A16.
Aziz, M. A., Atta, H., Mahfouz, S., Fouad, H., Roshdy, N., Ahmed, H., Rashed, L., Sabry, D., Hassouna, A., & Hasan, N. (2007). Therapeutic potential of bone marrow-derived mesenchymal stem cells on experimental liver fibrosis. Clinical Biochemistry, 40(12), 893–899.
Aziz, M. A., Atta, H., Roshdy, N. K., Ahmed, H. H., Rashed, L. A., Obaia, E., Sabry, D., & Ahmed, A. (2010). Role of SDF-1/CXCR4 axis in stem cell homing in the mouse model of induced lung fibrosis. International Journal Biotechnology Biochemistry, 6(4), 625–644.
Ohkawa, H., Ohishi, N., & Yagi, K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry, 95(2), 334–340.
Beutler, E., Duron, O., & Kelly, B. M. (1963). Improved method for the determination of blood glutathione. The Journal of Laboratory and Clinical Medicine, 61, 882–888.
Mortezaee, K., N. Khanlarkhani, F. Sabbaghziarani, S. Nekoonam, J. Majidpoor, A. Hosseini, P. Pasbakhsh, I.R. Kashani, and A. Zendedel, (2017). Preconditioning with melatonin improves therapeutic outcomes of bone marrow-derived mesenchymal stem cells in targeting liver fibrosis induced by CCl4. Cell and Tissue Research, p. 1–10.
Yi, X., Li, X., Zhou, Y., Ren, S., Wan, W., Feng, G., & Jiang, X. (2014). Hepatocyte growth factor regulates the TGF-β1-induced proliferation, differentiation and secretory function of cardiac fibroblasts. International Journal of Molecular Medicine, 34(2), 381–390.
Dury, R. A., & A.E. W. (1980). Histological Techniques (5th ed.pp. 27–29). Oxford: N. Y., Toronto.
Liu, Y. (2006). Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney International, 69(2), 213–217.
Rangan, G. K., Pippin, J. W., Coombes, J. D., & Couser, W. G. (2005). C5b-9 does not mediate chronic tubulointerstitial disease in the absence of proteinuria. Kidney International, 67(2), 492–503.
Rangan, G. K., Pippin, J. W., & Couser, W. G. (2004). C5b-9 regulates peritubular myofibroblast accumulation in experimental focal segmental glomerulosclerosis. Kidney International, 66(5), 1838–1848.
Pan, H., Shen, Z., Mukhopadhyay, P., Wang, H., Pacher, P., Qin, X., & Gao, B. (2009). Anaphylatoxin C5a contributes to the pathogenesis of cisplatin-induced nephrotoxicity. American journal of physiology Renal Physiology, 296(3), F496–F504.
Shalaby, R. H., Rashed, L. A., Ismaail, A. E., Madkour, N. K., & Elwakeel, S. H. (2014). Hematopoietic stem cells derived from human umbilical cord ameliorate cisplatin-induced acute renal failure in rats. American journal of stem cells, 3(2), 83–96.
Elsherbiny, N. M., Eladl, M. A., & Al-Gayyar, M. M. (2016). Renal protective effects of arjunolic acid in a cisplatin-induced nephrotoxicity model. Cytokine, 77, 26–34.
Bayomi, H. S., Elsherbiny, N. M., El-Gayar, A. M., & Al-Gayyar, M. M. (2013). Evaluation of renal protective effects of inhibiting TGF-beta type I receptor in a cisplatin-induced nephrotoxicity model. European Cytokine Network, 24(4), 139–147.
Lawrence, T. (2009). The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor Perspectives in Biology, 1(6), a001651.
Raju, N., Sakthivel, K. M., Kannan, N., Prabhu, V. V., & Guruvayoorappan, C. (2015). Cuscuta chinensis ameliorates immunosuppression and urotoxic effect of cyclophosphamide by regulating cytokines-GM-CSF and TNF-alpha. Applied Biochemistry and Biotechnology, 176(3), 742–757.
Sung, M. J., Kim, D. H., Jung, Y. J., Kang, K. P., Lee, A. S., Lee, S., Kim, W., Davaatseren, M., Hwang, J. T., Kim, H. J., Kim, M. S., Kwon, D. Y., & Park, S. K. (2008). Genistein protects the kidney from cisplatin-induced injury. Kidney International, 74(12), 1538–1547.
Ramesh, G., Zhang, B., Uematsu, S., Akira, S., & Reeves, W. B. (2007). Endotoxin and cisplatin synergistically induce renal dysfunction and cytokine production in mice. American Journal of Physiology Renal Physiology, 293(1), F325–F332.
Ramesh, G., Kimball, S. R., Jefferson, L. S., & Reeves, W. B. (2007). Endotoxin and cisplatin synergistically stimulate TNF-alpha production by renal epithelial cells. American Journal of Physiology Renal Physiology, 292(2), F812–F819.
Sanchez-Gonzalez, P. D., Lopez-Hernandez, F. J., Lopez-Novoa, J. M., & Morales, A. I. (2011). An integrative view of the pathophysiological events leading to cisplatin nephrotoxicity. Critical Reviews in Toxicology, 41(10), 803–821.
Boor, P., Sebekova, K., Ostendorf, T., & Floege, J. (2007). Treatment targets in renal fibrosis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association, 22(12), 3391–3407.
Feld, S. M., Hirschberg, R., Artishevsky, A., Nast, C., & Adler, S. G. (1995). Insulin-like growth factor I induces mesangial proliferation and increases mRNA and secretion of collagen. Kidney International, 48(1), 45–51.
Johnson, D. W., Saunders, H. J., Brew, B. K., Ganesan, A., Baxter, R. C., Poronnik, P., Cook, D. I., Gyory, A. Z., Field, M. J., & Pollock, C. A. (1997). Human renal fibroblasts modulate proximal tubule cell growth and transport via the IGF-I axis. Kidney International, 52(6), 1486–1496.
Wang, S., Denichilo, M., Brubaker, C., & Hirschberg, R. (2001). Connective tissue growth factor in tubulointerstitial injury of diabetic nephropathy. Kidney International, 60(1), 96–105.
Jones, J. I., Gockerman, A., Busby Jr., W. H., Camacho-Hubner, C., & Clemmons, D. R. (1993). Extracellular matrix contains insulin-like growth factor binding protein-5: potentiation of the effects of IGF-I. The Journal of Cell Biology, 121(3), 679–687.
Kuemmerle, J. F. (1997). Autocrine regulation of growth in cultured human intestinal muscle by growth factors. Gastroenterology, 113(3), 817–824.
Zimmermann, E. M., Li, L., Hou, Y. T., Cannon, M., Christman, G. M., & Bitar, K. N. (1997). IGF-I induces collagen and IGFBP-5 mRNA in rat intestinal smooth muscle. The American Journal of Physiology, 273(4 Pt 1), G875–G882.
Doi, T., Striker, L. J., Gibson, C. C., Agodoa, L. Y., Brinster, R. L., & Striker, G. E. (1990). Glomerular lesions in mice transgenic for growth hormone and insulinlike growth factor-I. I. Relationship between increased glomerular size and mesangial sclerosis. The American Journal of Pathology, 137(3), 541–552.
Mathews, L. S., Hammer, R. E., Behringer, R. R., D'Ercole, A. J., Bell, G. I., Brinster, R. L., & Palmiter, R. D. (1988). Growth enhancement of transgenic mice expressing human insulin-like growth factor I. Endocrinology, 123(6), 2827–2833.
Quaife, C. J., Mathews, L. S., Pinkert, C. A., Hammer, R. E., Brinster, R. L., & Palmiter, R. D. (1989). Histopathology associated with elevated levels of growth hormone and insulin-like growth factor I in transgenic mice. Endocrinology, 124(1), 40–48.
Liu, Y., Sun, A. M., & Dworkin, L. D. (1998). Hepatocyte growth factor protects renal epithelial cells from apoptotic cell death. Biochemical and Biophysical Research Communications, 246(3), 821–826.
Yo, Y., Morishita, R., Nakamura, S., Tomita, N., Yamamoto, K., Moriguchi, A., Matsumoto, K., Nakamura, T., Higaki, J., & Ogihara, T. (1998). Potential role of hepatocyte growth factor in the maintenance of renal structure: anti-apoptotic action of HGF on epithelial cells. Kidney International, 54(4), 1128–1138.
Liu, Y. (2002). Hepatocyte growth factor and the kidney. Current Opinion in Nephrology and Hypertension, 11(1), 23–30.
Matsumoto, K., & Nakamura, T. (2001). Hepatocyte growth factor: renotropic role and potential therapeutics for renal diseases. Kidney International, 59(6), 2023–2038.
Hu, M. C., Kuro-o, M., & Moe, O. W. (2010). Klotho and kidney disease. Journal of Nephrology, 23(Suppl 16), S136–S144.
Schnaper, H. W., Jandeska, S., Runyan, C. E., Hubchak, S. C., Basu, R. K., Curley, J. F., Smith, R. D., & Hayashida, T. (2009). TGF-beta signal transduction in chronic kidney disease. Frontiers in Bioscience, 14, 2448–2465.
Barrera-Chimal, J., Perez-Villalva, R., Ortega, J. A., Sanchez, A., Rodriguez-Romo, R., Durand, M., Jaisser, F., & Bobadilla, N. A. (2015). Mild ischemic injury leads to long-term alterations in the kidney: amelioration by spironolactone administration. International Journal of Biological Sciences, 11(8), 892–900.
Oh, Y. (2012). The insulin-like growth factor system in chronic kidney disease: pathophysiology and therapeutic opportunities. Kidney Research and Clinical Practice, 31(1), 26–37.
Alexandre, C. S., Volpini, R. A., Shimizu, M. H., Sanches, T. R., Semedo, P., Di Jura, V. L., Camara, N. O., Seguro, A. C., & Andrade, L. (2009). Lineage-negative bone marrow cells protect against chronic renal failure. Stem Cells, 27(3), 682–692.
Noronha, I. L., Fujihara, C. K., & Zatz, R. (2002). The inflammatory component in progressive renal disease—are interventions possible? Nephrology Dialysis Transplantation, 17(3), 363–368.
Fujihara, C. K., Malheiros, D. M. A. C., Zatz, R., & de Lourdes Noronha, I. (1998). Mycophenolate mofetil attenuates renal injury in the rat remnant kidney. Kidney International, 54(5), 1510–1519.
Kalluri, R., & Weinberg, R. A. (2009). The basics of epithelial-mesenchymal transition. The Journal of Clinical Investigation, 119(6), 1420.
Strutz, F. M. (2009). EMT and proteinuria as progression factors. Kidney International, 75(5), 475–481.
Humphreys, B.D. and J.V. Bonventre, (2008). Mesenchymal stem cells in acute kidney injury. Annual Review of Medicine. 59.
Villanueva, S., Céspedes, C., & Vio, C. P. (2006). Ischemic acute renal failure induces the expression of a wide range of nephrogenic proteins. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 290(4), R861–R870.
Duffield, J. S., & Bonventre, J. V. (2005). Kidney tubular epithelium is restored without replacement with bone marrow-derived cells during repair after ischemic injury. Kidney International, 68(5), 1956–1961.
Higgins, D. F., Kimura, K., Bernhardt, W. M., Shrimanker, N., Akai, Y., Hohenstein, B., Saito, Y., Johnson, R. S., Kretzler, M., & Cohen, C. D. (2007). Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. The Journal of Clinical Investigation, 117(12), 3810.
Inumaru, J., Nagano, O., Takahashi, E., Ishimoto, T., Nakamura, S., Suzuki, Y., Niwa, S. I., Umezawa, K., Tanihara, H., & Saya, H. (2009). Molecular mechanisms regulating dissociation of cell–cell junction of epithelial cells by oxidative stress. Genes to Cells, 14(6), 703–716.
Acknowledgements
We acknowledge the great help provided by Prof. Dr. Abdelmoniem Ali, Professor of Pathology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt, concerning the histopathological sector of the study.
Funding
There was no source of funding or grant from any organization to cover the study expenses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare that there is no competing interest.
Rights and permissions
About this article
Cite this article
Elseweidy, M.M., Askar, M.E., Elswefy, S.E. et al. Nephrotoxicity Induced by Cisplatin Intake in Experimental Rats and Therapeutic Approach of Using Mesenchymal Stem Cells and Spironolactone. Appl Biochem Biotechnol 184, 1390–1403 (2018). https://doi.org/10.1007/s12010-017-2631-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2631-0