Abstract
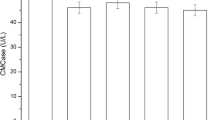
A halophilic cellulase-producing bacterium was isolated from a sediment sample collected from Lake Qarun (Fayoum Province, Egypt). Molecular identification based on 16S rDNA amplification and sequencing revealed 99% homology with Halobacillus sp. and hence was designated as Halobacillus sp. QLS 31. Medium composition and culture conditions were optimized for enhancing the production of cellulase enzyme using the Plackett-Burman statistical design. Ten variables were evaluated for their influence on cellulase production. Carboxymethyl cellulose (CMC), zinc sulfate (ZnSO4), and inoculum size were found to exert a significant effect on cellulase productivity by Halobacillus sp. QLS 31. The maximum specific activity of cellulase enzyme was 48.08 U/mg. Following the predicted conditions, a 7.5-fold increase in cellulase specific activity (175.47 U/mg) was achieved compared to the basal medium (23.19 U/mg) under the following optimized conditions: temperature (30 °C), fermentation time (2 days ), pH value (9), CMC concentration (1%), inoculum size (1%), yeast extract concentration (0.1%), ammonium sulfate ((NH3)2SO4) concentration (0.1%), sodium chloride (NaCl) concentration (20%), and metal inducers: ZnSO4 (0.1%) and Ca/Mg ratio (0.01%). Thus, the results of this study provide an important basis for more efficient, cheap industrial cellulase production from halophilic Halobacillus sp. QLS 31.



Similar content being viewed by others
References
Acharya, S., & Chaudhary, A. (2012). Bioprospecting thermophiles for cellulase production: a review. Brazilian Journal of Microbiology., 43, 844–856.
Phitsuwan, P., Laohakunjit, N., Kerdchoechuen, O., Kyu, K. L., & Ratanakhanokchai, K. (2012). Present and potential applications of cellulases in agriculture, biotechnology and bioenergy. Folia Biologica, 58, 163–176.
Venkatachalam, S., Sivaprakash, M., Gowdaman, V., & Prabagaran, S. R. (2014). Bioprospecting of cellulase producing extremophilic bacterial isolates from India. British Microbiology Research Journal., 4, 142–154.
Sethi, S., Datta, A., Lal Gupta, B., & Gupta, S. (2013). Optimization of cellulase production from bacteria isolated from soil. ISRN biotechnology., 2013, 1–7.
Sadhu, S., & Maiti, T. K. (2013). Cellulase production by bacteria: a review. British Microbiology Research Journal., 3, 235–258.
Immanuel, G., Dhanusha, R., Prema, P., & Palavesam, A. (2006). Effect of different growth parameters on endoglucanase enzyme activity by bacteria isolated from coir retting effluents of estuarine environment. International journal of environmental science and technology., 3, 25–34.
Ladeira, A., Cruz, E., Delatorre, B., Barbosa, B., & Martins, M. (2015). Cellulase production by thermophilic Bacillus sp. SMIA-2 and its detergent compatibility. Electronic journal of biotechnology., 18, 110–115.
Madern, D., Ebel, C., & Zaccai, G. (2000). Halophilic adaptation of enzymes. Extremophiles, 4, 91–98.
Dassarma, P., Coker, J. A., Huse, V., & Dassarma, S. (2010). Halophiles, industrial application. Encyclopedia of Industrial Biotechnology, 7, 1–10.
Delgado-García, M. V., Blanca, A., Cristóbal, N., Contreras-Esquivel, J. C., & Rodríguez-Herrera, R. (2012). Halophilic hydrolases as a new tool for the biotechnological industries. Journal of the Science of Food and Agriculture., 92, 2575–2580.
Shivanand, P., Mugeraya, G., & Kumar, A. (2012). Utilization of renewable agricultural residues for the production of extracellular halostable cellulase from newly isolated Halomonas sp. strain PS47. Annals of Microbiology., 63, 1257–1263.
Darwish, S. M., El-Bahi, S. M., Sroor, A. T., & Arhoma, N. F. (2013). Natural radioactivity assessment and radiological hazards in soils from Qarun Lake and Wadi El Rayan in Faiyum, Egypt. Open Journal of Soil Science., 3, 289–296.
Elbanna, K., Ibrahim, I. M., & Revol-Junelles, A. M. (2015). Purification and characterization of halo-alkali-thermophilic protease from Halobacterium sp. strain HP25 isolated from raw salt, Lake Qarun, Fayoum, Egypt. Extremophiles, 19, 763–774.
Mădălin, E., Roxana, C., Simona, M., Gabriela, P., & Lucia, D. (2009). Extracellular hydrolytic enzymes of halophilic bacteria isolated from a subterranean rock salt crystal. Romanian Biotechnological Letters., 14, 4658–4664.
Chand, R., Richa, K. Æ., Dhar, H., Dutt, Æ. S., & Gulati, Æ. A. (2008). A rapid and easy method for the detection of microbial cellulases on agar plates using gram’s iodine. Current Microbiology., 57, 503–507.
Thompson, J. D., Higgins, D. G., & Gibson, T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research., 22, 4673–4680.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., & Kumar, S. (2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution., 28, 2731–2739.
Reddy, C. R. K., Trivedi, N., Gupta, V., Kumar, M., Kumari, P., & Jha, B. (2011). An alkali-halotolerant cellulase from Bacillus flexus isolated from green seaweed Ulva lactuca. Carbohydrate Polymers., 83, 891–897.
Ghose, T. K. (1987). Measurement of cellulase activities. Pure & Applied chemistry., 59, 257–268.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry., 254, 248–254.
Brereton, R. G. (2003). Chemometrics: data analysis for the laboratory and chemical plant. New York: Wiley.
Oren, A. (2010). Industrial and environmental applications of halophilic microorganisms. Environmental technology., 31, 825–834.
Mesbah, N. M., & Wiegel, J. (2011). Halophiles exposed concomitantly to multiple Stressors : adaptive mechanisms of halophilic alkalithermophiles. In E. Roine & H. M. Oksanen (Eds.), Halophiles and hypersaline environments (pp. 249–273). Berlin Heidelberg: Springer.
Dalmaso, G., Ferreira, D., & Vermelho, A. (2015). Marine extremophiles: a source of hydrolases for biotechnological applications. Marin Drugs., 13, 1925–1965.
Raddadi, N., Cherif, A., Daffonchio, D., Neifar, M., & Fava, F. (2015). Biotechnological application of extremophiles, extremozymes and extremolytes. Applied Microbiology and Biotechnology., 99, 7907–7913.
Zhang, T., et al. (2011). Identification of a haloalkaliphilic and thermostable cellulase with improved ionic liquid tolerance. Green Chemistry., 13, 2083–2090.
Cao, G., Zhao, L., Wang, A., Wang, Z., & Ren, N. (2014). Single-step bioconversion of lignocellulose to hydrogen using novel moderately thermophilic bacteria. Biotechnology for biofuels., 7, 82–90.
Deka, D., Bhargavi, P., Sharma, A., Goyal, D., Jawed, M., & Goyal, A. (2011). Enhancement of cellulase activity from a new strain of Bacillus subtilis by medium optimization and analysis with various cellulosic substrates. Enzyme research., 2011, 1–8.
Annamalai, N., & Veeramuthu, M. (2014). Enzymatic saccharification of pretreated rice straw by cellulase produced from Bacillus carboniphilus CAS 3 utilizing lignocellulosic wastes through statistical optimization. Biomass and Bioenergy, 68, 151–160.
Shanmughapriya, S., Kiran, G. S., Selvin, J., Thomas, T. A., & Rani, C. (2010). Optimization, purification, and characterization of extracellular mesophilic alkaline cellulase from sponge-associated Marinobacter sp. MSI032. Applied Biochemistry and Biotechnology., 162, 625–640.
Vasudeo, Z., & Lew, C. (2011). Optimization of culture conditions for production of cellulase by a thermophilic Bacillus strain. Journal of Chemistry and Chemical Engineering., 5, 521–527.
Asha, B. M., & Sakthivel, N. (2014). Production, purification and characterization of a new cellulase from Bacillus subtilis that exhibit halophilic, alkalophilic and solvent-tolerant properties. Annals of Microbiology., 64, 1839–1848.
Lugani, Y., Singla, R., & Sooch, B. S. (2015). Optimization of cellulase production from newly isolated Bacillus sp. Y3. Journal of Bioprocessing & Biotechniques., 5, 3–8.
Kazemi, A., Rasoul-Amini, S., Shahbazi, M., Safari, A., & Ghasemi, Y. (2013). Isolation, identification, and media optimization of high-level cellulase production by Bacillus sp. Bccs a3, in a fermentation system using response surface methodology. Preparative Biochemistry and Biotechnology., 44, 107–118.
Das, A., Paul, T., Halder, S. K., Maity, C., Mohapatra, P., Das, K., Pati, B. R., & Mondal, K. C. (2013). Study on regulation of growth and biosynthesis of cellulolytic enzymes from newly isolated Aspergillus fumigatus ABK9. Polish Journal of Microbiology, 62, 31–43.
Shankar, T., & Isaiarasu, L. (2011). Cellulase production by Bacillus pumilus EWBCM1 under varying cultural conditions. Middle-East Journal of Scientific Research., 8, 40–45.
Liang, Y. L., Zhang, Z., Wu, M., Wu, Y., & Feng, J. X. (2014). Isolation, screening, and identification of cellulolytic bacteria from natural reserves in the subtropical region of China and optimization of cellulase production by Paenibacillus terrae ME27-1. BioMed Research International., 2014, 1–13.
Shadrin, N. V., Anufriieva, E. V., Goher, M. E., & Ragab, E. (2016). Long-term changes of physicochemical parameters and benthos in Lake Qarun (Egypt): can we make a correct forecast of ecosystem future ? Knowledge and Management of Aquatic Ecosystems., 417, 1–18.
Singh, J., & Kaur, P. (2012). Optimization of process parameters for cellulase production from Bacillus sp . JS14 in solid substrate fermentation using response surface methodology. Brazilian Archives of Biology and Technology., 55, 505–512.
Gaur, R., & Tiwari, S. (2015). Isolation, production, purification and characterization of an organic-solvent-thermostable alkalophilic cellulase from Bacillus vallismortis RG-07. BMC biotechnology., 15, 19–41.
Goyal, V., Mittal, A., Bhuwal, A. K., Singh, G., Yadav, A., & Aggarwal, N. K. (2014). Parametric optimization of cultural conditions for carboxymethyl cellulase production using pretreated rice straw by Bacillus sp. 313SI under stationary and shaking conditions. Biotechnology Research International., 2014, 1–7.
Das, A., Bhattacharya, S., & Murali, L. (2010). Production of cellulase from thermophilic Bacillus sp. isolated from cow dung. American-Eurasian Journal of Agricultural and Environmental Sciences, 8, 685–691.
Wang, C., Hsieh, Y., Ng, C., Chan, H., Lin, H., Tzeng, W., & Shyu, Y. (2009). Enzyme and microbial technology purification and characterization of a novel halostable cellulase from Salinivibrio. Enzyme and Microbial Technology., 44, 373–379.
Li, X., Wang, H. L., Li, T., & Yu, H. Y. (2012). Purification and characterization of an organic solvent-tolerant alkaline cellulase from a halophilic isolate of Thalassobacillus. Biotechnology Letters., 34, 1531–1536.
Li, X., & Yu, H. Y. (2012). Purification and characterization of an organic-solvent-tolerant cellulase from a halotolerant isolate, Bacillus sp. L1. Journal of Industrial Microbiology and Biotechnology., 39, 117–1124.
Balasubramanian, N., & Simões, N. (2014). Bacillus pumilus S124A carboxymethyl cellulase; a thermo stable enzyme with a wide substrate spectrum utility. International Journal of Biological Macromolecules., 67, 132–139.
Singh, K., Richa, K., Bose, H., Karthik, L., Kumar, G., Bhaskara, R., & Kokati, V. (2014). Statistical media optimization and cellulase production from marine Bacillus VITRKHB. 3 Biotech, 4, 591–598.
Assareh, R., Shahbani, Z. H., Akbari, N. K., Aminzadeh, S., & Bakhshi, K. G. (2012). Characterization of the newly isolated Geobacillus sp. T1, the efficient cellulase-producer on untreated barley and wheat straws. Bioresource Technology., 120, 99–105.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Korany, A.H., Ali, A.E., Essam, T.M. et al. Optimization of Cellulase Production by Halobacillus sp. QLS 31 Isolated from Lake Qarun, Egypt. Appl Biochem Biotechnol 183, 189–199 (2017). https://doi.org/10.1007/s12010-017-2438-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2438-z