Abstract
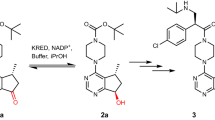
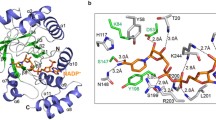
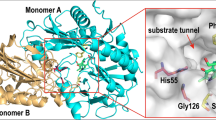
(S)-N-Boc-3-hydroxypiperidine ((S)-NBHP) is a key pharmaceutical intermediate and the chiral source in synthesizing Imbruvica, which is a newly approved drug in lymphoma therapy by targeting Bruton’s tyrosine kinase (BTK). Current chemical synthesis of (S)-NBHP suffered from the need of noble metal catalyst and low yield. The single reported bioconversion of (S)-NBHP was achieved by using recombinant ketoreductase, but enzyme sequence was kept confidential and the catalytic process suffered from the thermodeactivation and substrate inhibition. In the current study, we presented a thermostable aldo-keto reductase (AKR)—AKR-43—which showed high activity toward N-Boc-3-piperidone (NBP) to produce (S)-NBHP, high enantioselectivity, and no substrate inhibition. The molecular simulations demonstrated the structural rationale for the enantioselectivity of AKR-43 toward NBP and supported the classic ordered two-step catalytic mechanism. The catalytic process was achieved by using glucose dehydrogenase (GDH) for cofactor recycling, and the optimal reaction conditions were determined to be 30 °C and pH 7.5. Within a reaction time of 16 h, the 16 % substrate concentration (w/w), over 99 % ee and under 3.5 % of enzyme loading (w/w) characterized a high efficiency process with promising industrial values.






Similar content being viewed by others
References
Wijdeven, M. A., Willemsen, J., & Rutjes, F. P. J. T. (2010). The 3-hydroxypiperidine skeleton: key element in natural product synthesis. European Journal of Organic Chemistry, 15, 2831–2844.
Ju, X., Tang, Y. Y., Liang, X. L., Hou, M. Q., Wan, Z. H., & Tao, J. H. (2014). Development of a biocatalytic process to prepare (S)-N-boc-3-hydroxypiperidine. Organic Process Research & Development, 18, 827–830.
Zechel, D. L., Boraston, A. B., Gloster, T., Boraston, C. M., Macdonald, J. M., Tilbrook, D. M. G., Stick, R. V., & Davies, G. J. (2003). Iminosugar glycosidase inhibitors: structural and thermodynamic dissection of the binding of isofagomine and 1-deoxynojirimycin to β-glucosidases. Journal of the American Chemical Society, 125, 14313–14323.
Heightman, T. D., & Vasella, A. T. (1999). Recent insights into inhibition, structure, and mechanism of configuration-retaining glycosidases. Angewandte Chemie International Edition, 38, 750–770.
Butters, T. D., Dwek, R. A., & Platt, F. M. (2000). Inhibition of glycosphingolipid biosynthesis: application to lysosomal storage disorders. Chemical Reviews, 100, 4683–4696.
Chiou, W. H., Lin, G. H., & Liang, C. W. (2010). Facile syntheses of enantiopure 3-hydroxypiperidine derivatives and 3-hydroxypipecolic acids. Journal of Organic Chemistry, 75, 1748–1751.
Aboul-Enein, H. Y., Serignese, V., Minguillon, C., & Oliveros, L. (1997). Enantioselective separation of several piperidine-2, 6-diones on a covalently bonded cellulose 3, 5-dimethylphenyl carbamate/10-undecenoate chiral selector. Biomedical Chromatography, 11, 303–306.
Ali, I., Naim, L., Ghanem, A., & Aboul-Enein, H. Y. (2006). Chiral separations of piperidine-2, 6-dione analogues on Chiralpak IA and Chiralpak IB columns by using HPLC. Talanta, 69, 1013–1017.
Ortiz, A., Young, I. S., Sawyer, J. R., Hsiao, Y., Singh, A., Sugiyama, M., Corbett, R. M., Chau, M., Shi, Z., & Conlon, D. A. (2012). Synthetic approaches to a chiral 4-amino-3-hydroxy piperidine with pharmaceutical relevance. Organic & Biomolecular Chemistry, 10, 5253–5257.
Poerwono, H., Higashiyama, K., & Takahashi, H. (1998). Stereocontrolled syntheses of piperidine derivatives using diastereoselective reactions of chiral 1,3-oxazolidines with grignard reagents: asymmetric syntheses of the pinidine enantiomers. The Journal of Organic Chemistry, 63, 2711–2714.
Willert, M., & Bols, M. (1998). A study of Baker’s yeast reduction of piperidone-carboxylates. Acta Chemica Scandinavica, 52, 461–468.
Lacheretz, R., Pardo, D. G., & Cossy, J. (2009). Daucus carota mediated-reduction of cyclic 3-oxo-amines. Organic Letters, 11, 1245–1248.
Liu, N., Hoogendoorn, S., van de Kar, B., Kaptein, A., Barf, T., Driessen, C., Filippov, D. V., van der Marel, G. A., van der Stelt, M., & Overkleeft, H. S. (2015). Direct and two-step bioorthogonal probes for Bruton’s tyrosine kinase based on ibrutinib: a comparative study. Organic & Biomolecular Chemistry, 13, 5147–5157.
Amat, M., Llor, N., Huguet, M., Molins, E., Espinosa, E., & Bosch, J. (2001). Unprecedented oxidation of a phenylglycinol-derived 2-pyridone: enantioselective synthesis of polyhydroxypiperidines. Organic Letters, 3, 3257–3260.
Hou, H., Li, R., Wang, X., Yuan, Z., Liu, X., Chen, Z., & Xu, X. (2015). Crystallographic analysis of a novel aldo-keto reductase from Thermotoga maritima in complex with NADP(+). Acta Crystallographica Section F-Structural Biology and Crystallization Communications, 71, 847–855.
Wang, Z., Ling, B., Zhang, R., Suo, Y., Liu, Y., Yu, Z., & Liu, C. (2009). Docking and molecular dynamics studies toward the binding of new natural phenolic marine inhibitors and aldose reductase. Journal of Molecular Graphics and Modelling, 28, 162–169.
Morris, G. M., Goodsell, D. S., Halliday, R. S., Huey, R., Hart, W. E., Belew, R. K., & Olson, A. J. (1998). Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. Journal of Computational Chemistry, 19, 1639–1662.
Case, D. A., Cheatham, T. E., Darden, T., Gohlke, H., Luo, R., Merz, K. M., Onufriev, A., Simmerling, C., Wang, B., & Woods, R. J. (2005). The Amber biomolecular simulation programs. Journal of Computational Chemistry, 26, 1668–1688.
Götz, A. W., Williamson, M. J., Xu, D., Poole, D., Le Grand, S., & Walker, R. C. (2012). Routine microsecond molecular dynamics simulations with AMBER on GPUs. 1. Generalized born. Journal of Chemical Theory and Computation, 8, 1542–1555.
Salomon-Ferrer, R., Götz, A. W., Poole, D., Le Grand, S., & Walker, R. C. (2013). Routine microsecond molecular dynamics simulations with AMBER on GPUs. 2. Explicit solvent particle mesh Ewald. Journal of Chemical Theory and Computation, 9, 3878–3888.
Ponder, J. W., & Case, D. A. (2003). Force fields for protein simulations. Advances in Protein Chemistry, 66, 27–86.
Wang, Z., & Liu, J. P. (2015). Characterization of potassium binding with human telomeres. Clinical and Experimental Pharmacology and Physiology, 42, 902–909.
Ma, Y.-H., Lv, D.-Q., Zhou, S., Lai, D.-Y., & Chen, Z.-M. (2013). Characterization of an aldo-keto reductase from Thermotoga maritima with high thermostability and a broad substrate spectrum. Biotechnology Letters, 35, 757–762.
Kratzer, R., Wilson, D. K., & Nidetzky, B. (2006). Catalytic mechanism and substrate selectivity of aldo-keto reductases: insights from structure-function studies of Candida tenuis xylose reductase. IUBMB Life, 58, 499–507.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Mengyan He and Shuo Zhou contributed equally to this work.
Electronic supplementary material
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
He, M., Zhou, S., Cui, M. et al. Efficient Preparation of (S)-N-Boc-3-Hydroxylpiperidine Through Bioreduction by a Thermostable Aldo-KetoReductase. Appl Biochem Biotechnol 181, 1304–1313 (2017). https://doi.org/10.1007/s12010-016-2285-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2285-3