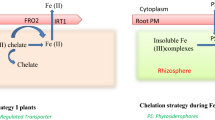
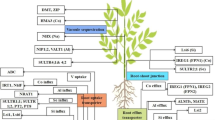
Abstract
The essentiality of 14 mineral elements so far have been reported in plant nutrition. Eight of these elements were known as micronutrients due to their lower concentrations in plants (usually ≤100 mg/kg/dw). However, it is still challenging to mention an exact number of plant micronutrients since some elements have not been strictly proposed yet either as essential or beneficial. Micronutrients participate in very diverse metabolic processes, including from the primary and secondary metabolism to the cell defense, and from the signal transduction to the gene regulation, energy metabolism, and hormone perception. Thus, the attempt to understand the molecular mechanism(s) behind their transport has great importance in terms of basic and applied plant sciences. Moreover, their deficiency or toxicity also caused serious disease symptoms in plants, even plant destruction if not treated, and many people around the world suffer from the plant-based dietary deficiencies or metal toxicities. In this sense, shedding some light on this issue, the 13 mineral elements (Fe, B, Cu, Mn, Mo, Si, Zn, Ni, Cl, Se, Na, Al, and Co), required by plants at trace amounts, has been reviewed with the primary focus on the transport proteins (transporters/channels) in plant roots. So, providing the compiled but extensive information about the structural and functional roles of micronutrient transport genes/proteins in plant roots.
Similar content being viewed by others
References
Barker, A. V., & Pilbeam, D. J. (Eds.). (2015). Handbook of plant nutrition. CRC press.
Mengel, K., Kosegarten, H., Kirkby, E. A., & Appel, T. (Eds.) (2001). Principles of plant nutrition. Springer Science and Business, Media.
Maathuis, F. J. M. (2013). Plant mineral nutrients: methods and protocols. New York: Humana Press.
Maathuis, F. J., & Diatloff, E. (2013). Roles and functions of plant mineral nutrients. Plant Mineral Nutrients: Methods and Protocols, 1–21.
Marschner, H. (1995). Functions of mineral nutrients: macronutrients. Mineral nutrition of higher plants, 2, Academic Press, London, 379–396.
Grotz, N., Fox, T., Connolly, E., Park, W., Guerinot, M. L., & Eide, D. (1998). Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proceedings of the National Academy of Sciences, 95, 7220–7224.
Underwood, E. (2012). Trace elements in human and animal nutrition 4e. Elsevier.
Marschner, P. (2012). Marschner’s mineral nutrition of higher plants. Boston, MA: Academic Press.
Tavsan, Z., & Kayali, H. A. (2013). The effect of iron and copper as an essential nutrient on mitochondrial electron transport system and lipid peroxidation in Trichoderma harzianum. Applied Biochemistry and Biotechnology, 170(7), 1665–1675.
Vatansever, R., Filiz, E., & Ozyigit, I. I. (2015). Genome-wide analysis of iron-regulated transporter 1 (IRT1) genes in plants. Horticulture, Environment, and Biotechnology, 56(4), 516–523.
Vert, G., Grotz, N., Dédaldéchamp, F., Gaymard, F., Guerinot, M. L., Briat, J. F., et al. (2002). IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell Online, 14, 1223–1233.
Kobayashi, T., & Nishizawa, N. K. (2012). Iron uptake, translocation, and regulation in higher plants. Annual Review of Plant Biology, 63, 131–152.
Tombuloğlu, H., Ablazov, A., & Filiz, E. (2016). Genome-wide analysis of response to low sulfur (LSU) genes in grass species and expression profiling of model grass species Brachypodium distachyon under S-deficiency. Turkish Journal of Biology. doi:10.3906/biy-1508-32.
López-Millán, A. F., Grusak, M. A., Abadía Bayona, A., & Abadía Bayona, J. (2013). Iron deficiency in plants: an insight from proteomic approaches. Frontiers in Plant Science, 4, 254.
Kim, S. A., & Guerinot, M. L. (2007). Mining iron: iron uptake and transport in plants. FEBS Letters, 581, 2273–2280.
Briat, J. F., & Lobr’eaux, S. (1997). Iron transport and storage in plants. Trends in Plant Sciences, 2, 187–193.
Briat, J. F., Dubos, C., & Gaymard, F. (2015). Iron nutrition, biomass production, and plant product quality. Trends in Plant Science, 20(1), 33–40.
Römheld, V., & Marschner, H. (1986). Evidence for a specific uptake system for iron phytosiderophore in roots of grasses. Plant Physiology, 80, 175–180.
Higuchi, K., Suzuki, K., Nakanishi, H., Yamaguchi, H., Nishizawa, N. K., & Mori, S. (1999). Cloning of nicotianamine synthase genes, novel genes involved in the biosynthesis of phytosiderophores. Plant Physiology, 119, 471–479.
Takahashi, M., Yamaguchi, H., Nakanishi, H., Shioiri, T., Nishizawa, N. K., & Mori, S. (1999). Cloning two genes for nicotianamine aminotransferase, a critical enzyme in iron acquisition (strategy II) in graminaceous plants. Plant Physiology, 121, 947–956.
Bashir, K., Inoue, H., Nagasaka, S., Takahashi, M., Nakanishi, H., Mori, S., et al. (2006). Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants. Journal of Biological Chemistry, 43, 32395–32402.
Inoue, H., Kobayashi, T., Nozoye, T., Takahashi, M., Kakei, Y., Suzuki, K., et al. (2009). Rice OsYSL15 is an iron-regulated iron (III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. Journal of Biological Chemistry, 284, 3470–3479.
Lee, S., Chiecko, J. C., Kim, S. A., Walker, E. L., Lee, Y., Guerinot, M. L., et al. (2009). Disruption of OsYSL15 leads to iron inefficiency in rice plants. Plant Physiology, 150, 786–800.
Nozoye, T., Nagasaka, S., Kobayashi, T., Takahashi, M., Sato, Y., Sato, M., et al. (2011). Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. Journal of Biological Chemistry, 286, 5446–5454.
Thomine, S., & Vert, G. (2013). Iron transport in plants: better be safe than sorry. Current Opinion in Plant Biology, 16(3), 322–327.
Colangelo, E. P., & Guerinot, M. L. (2006). Put the metal to the petal: metal uptake and transport throughout plants. Current Opinion in Plant Biology, 9, 322–330.
Guerinot, M. L. (2000). The ZIP family of metal transporters. Biochimica et Biophysica Acta (BBA), 1465, 190–198.
Eng, B. H., Guerinot, M. L., Eide, D., & Saier Jr., M. H. (1998). Sequence analyses and phylogenetic characterization of the ZIP family of metal ion transport proteins. Journal of Membrane Biology, 166, 1–7.
Thomine, S., Wang, R., Ward, J. M., Crawford, N. M., & Schroeder, J. I. (2000). Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proceedings of the National Academy of Sciences, USA, 97, 4991–4996.
Nevo, Y., & Nelson, N. (2006). The NRAMP family of metal-iontransporters. Biochimica et Biophysica Acta (BBA), 1763, 609–620.
Xia, J., Yamaji, N., Kasai, T., & Ma, J. F. (2010). Plasma membrane-localized trans-Porter for aluminum in rice. Proceedings of the National Academy of Sciences, USA, 107, 18381–18385.
Cellier, M., Prive, G., Belouchi, A., Kwan, T., Rodrigues, V., Chia, W., et al. (1995). Nramp defines a family of membrane proteins. Proceedings of the National Academy of Sciences, USA, 92, 10089–10093.
Schaaf, G., Ludewig, U., Erenoglu, B. E., Mori, S., Kitahara, T., & Von Wiren, N. (2004). ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. Journal of Biological Chemistry, 279, 9091–9096.
Yen, M. R., Tseng, Y. H., & Saier Jr., M. H. (2001). Maize yellow Stripe1, an iron-phytosiderophore uptake transporter, is a member of the oligopeptide transporter (OPT) family. Microbiology, 147, 2881–2883.
Höreth, S., Detterbeck, A., Ahmadi, H., Pongrac, P., & Clemens, S. (2015). Molecular analysis of within-plant Zn mobility in a metal hyperaccumulator and a crop model system. In OF ABSTRACTS (p. 22).
Fox, T. C., & Guerinot, M. L. (1998). Molecular biology of cation transport in plants. Annual Review of Plant Biology, 49, 669–696.
Sadeghzadeh, B., & Rengel, Z. (2011). Zinc in soils and crop nutrition. The molecular and physiological basis of nutrient use efficiency in crops, John Wiley & Sons, Inc., pp. 335–375.
Maser, P., Thomine, S., Schroeder, J. I., Ward, J. M., Hirschi, K., Sze, H., et al. (2001). Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiology, 126, 1646–1667.
Lin, Y. F., Liang, H. M., Yang, S. Y., Boch, A., Clemens, S., Chen, C. C., et al. (2009). Arabidopsis IRT3 is a zinc-regulated and plasma membrane localized zinc/iron transporter. New Phytologist, 182, 392–404.
Assunção, A. G., Herrero, E., Lin, Y. F., Huettel, B., Talukdar, S., Smaczniak, C., Immink, R. H., Eldik, Mv., Fiers, M., Schat, H. et al. (2010). Arabidopsis thaliana transcription factors bZIP19 and bZIP23 regulate the adaptation to zinc deficiency. Proceedings of the National Academy of Sciences of the United States of America, 10296–10301.
Connolly, E. L., Fett, J. P., & Guerinot, M. L. (2002). Expression of the irt1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell, 14, 1347–1357.
Vert, G., Briat, J. F., & Curie, C. (2001). Arabidopsis irt2 gene encodes a root-periphery iron transporter. Plant Journal, 26, 181–189.
Moreau, S., Thomson, R. M., Kaiser, B. N., Trevaskis, B., Guerinot, M. L., Udvardi, M. K., et al. (2002). GmZIP1 encodes a symbiosis-specific zinc transporter in soybean. Journal of Biological Chemistry, 15, 4738–4746.
Ramesh, S. A., Shin, R., Eide, D. J., & Schachtman, D. P. (2003). Defferential metal selectivity and gene expression of two zinc transporters from rice. Plant Physiology, 133, 126–134.
Ishimaru, Y., Suzuki, M., Kobayashi, T., Takahashi, M., Nakanishi, H., Mori, S., et al. (2005). OsZIP4, a novel zinc-regulated zinc transporter in rice. Journal of Experimental Botany, 56, 3207–3214.
Lopez-Millan, A. F., Ellis, D. R., & Grusak, M. A. (2004). Identification and characterization of several new members of the zip family of metal ion transporters in Medicago truncatula. Plant Mol Biology, 54, 583–596.
Vatansever, R., Ozyigit, I. I., & Filiz, E. (2015). Comparative and phylogenetic analysis of zinc (Zn) transporter genes/proteins in plants. Turkish Journal of Biology. doi:10.3906/biy-1501-91.
Coudray, N., Zhang, Z., Clark, K. M., Ubarretxena, I., Beckstein, O., Dumont, M. E., et al. (2016). Structure of the borate transporter Bor1p by cryo-EM. Biophysical Journal, 110(3), 137–138.
Shen, Z. G., Liang, Y. C., & Shen, K. (1993). Effect of boron on the nitrate reductase-activity in oilseed rape plants. Journal of Plant Nutrition, 16, 1229–1239.
Camacho-Cristóbal, J. J., & González-Fontes, A. (2007). Boron deficiency decreases plasmalemma H+−ATPase expression and nitrate uptake, and promotes ammonium assimilation into asparagine in tobacco roots. Planta, 226, 443–451.
Bassil, E., Hu, H., & Brown, P. H. (2004). Use of phenylboronic acids to investigate boron function in plants. Possible role of boron in transvacuolar cytoplasmic strands and cell-to-wall adhesion. Plant Physiology, 136, 3383–3395.
Gonzalez-Fontes, A., Rexach, J., Navarro-Gochicoa, M. T., Herrera-Rodrıguez, M. B., Beato, V. M., Maldonado, J. M., et al. (2008). Is boron involved solely in structural roles in vascular plants? Plant Signaling and Behavior, 3, 24–26.
Ferrol, N., & Donaire, J. P. (1992). Effect of boron on plasma membrane proton extrusion and redox activity in sunflower cells. Plant Science, 86, 41–47.
Wang, Z. Y., Tang, Y. L., Zhang, F. S., & Wang, H. (1999). Effect of boron and low temperature on membrane integrity of cucumber leaves. Journal of Plant Nutrition, 22, 543–550.
Fleischer, A., O’Neill, M. A., & Ehwald, R. (1999). The pore size of nongraminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiology, 121, 829–838.
Ryden, P., Sugimoto-Shirasu, K., Smith, A. C., Findlay, K., Reiter, W. D., & McCann, M. C. (2003). Tensile properties of Arabidopsis cell walls depend on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes. Plant Physiology, 132, 1033–1040.
Camacho-Cristóbal, J. J., Anzelotti, D., & González-Fontes, A. (2002). Changes in phenolic metabolism of tobacco plants during short-term boron deficiency. Plant Physiology and Biochemistry, 40, 997–1002.
Tanaka, M., & Fujiwara, T. (2008). Physiological roles and transport mechanisms of boron: perspectives from plants. European Journal of Physiology, 456, 671–677.
Dannel, F., Pfeffer, H., & R¨omheld, V. (2002). Update on boron in higher plant-uptake, primary translocation and compartmentation. Plant Biology, 4, 193–204.
Takano, J., Noguchi, K., Yasumori, M., Kobayashi, M., Gajdos, Z., Miwa, K., et al. (2002). Arabidopsis boron transporter for xylem loading. Nature, 420, 337–340.
Takano, J., Wada, M., Ludewig, U., Schaaf, G., von Wir ´en, N., & Fujiwara, T. (2006). The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell, 18, 1498–1509.
Kruse, E., Uehlein, N., & Kaldenhoff, R. (2006). The aquaporins. Genome Biology, 7, 206.
Nakagawa, Y., Hanaoka, H., Kobayashi, M., Miyoshi, K., Miwa, K., & Fujiwara, T. (2007). Cell-type specificity of the expression of OsBOR1, a rice efflux boron transporter gene, is regulated in response to boron availability for efficient boron uptake and xylem loading. Plant Cell, 19, 2624–2635.
Hanaoka, H., Uraguchi, S., Takano, J., Tanaka, M., & Fujiwara, T. (2014). OsNIP3; 1, a rice boric acid channel, regulates boron distribution and is essential for growth under boron-deficient conditions. Plant Journal, 78, 890–902.
Takano, J., Miwa, K., & Fujiwara, T. (2008). Boron transport mechanisms: collaboration of channels and transporters. Trends in Plant Sciences, 13, 451–457.
Pérez-Castro, R., Kasai, K., Gainza-Cortés, F., Ruiz-Lara, S., Casaretto, J. A., Peña-Cortés, H., et al. (2012). VvBOR1, the grapevine ortholog of AtBOR1, encodes an efflux boron transporter that is differentially expressed throughout reproductive development of Vitis vinifera L. Plant and Cell Physiology, 53(2), 485–494.
Sun, J., Shi, L., Zhang, C., & Xu, F. (2012). Cloning and characterization of boron transporters in Brassica napus. Molecular Biology Reports, 39, 1963–1973.
Domingues, D. S., Maximino Leite, S. M., & Cazerta Farro, A. P. (2005). Boron transport in eucalyptus. 2. Identification in silico of a putative boron transporter for xylem loading in eucalypt. Genetics and Molecular Biology, 28, 625–629.
Chatterjee, M., Tabi, Z., Galli, M., Malcomber, S., Buck, A., Muszynski, M., et al. (2014). The boron efflux transporter ROTTEN EAR is required for maize inflorescence development and fertility. Plant Cell, 26, 2962–2977.
Durbak, A. R., Phillips, K. A., Pike, S., O’Neill, M. A., Mares, J., Gallavotti, A., et al. (2014). Transport of boron by the tassel-less1 aquaporin is critical for vegetative and reproductive development in maize. Plant Cell, 26, 2978–2995.
Leonard, A., Holloway, B., Guo, M., Rupe, M., Yu, G., Beatty, M., & Li, B. (2014). tassel-less1 encodes a boron channel protein required for inflorescence development in maize. Plant Cell Physiology, pcu036.
Adrees, M., Ali, S., Rizwan, M., Ibrahim, M., Abbas, F., Farid, M., et al. (2015). The effect of excess copper on growth and physiology of important food crops: a review. Environmental Science and Pollution Research, 22(11), 8148–8162.
Ng, I. S., Zheng, X., Wang, N., Chen, B. Y., Zhang, X., & Lu, Y. (2014). Copper response of Proteus hauseri based on proteomic and genetic expression and cell morphology analyses. Applied Biochemistry and Biotechnology, 173(5), 1057–1072.
Clemens, S. (2001). Molecular mechanisms of plant metal tolerance and homeostasis. Planta, 212, 475–486.
Huffman, D. L., & O’Halloran, T. V. (2001). Function, structure, and mechanism of intracellular copper trafficking proteins. Annual Review of Biochemistry, 70, 677–701.
Burkhead, J. L., Gogolin, R. K. A., Abdel-Ghany, S. E., Cohu, C. M., & Pilon, M. (2009). Copper homeostasis. New Phytologist, 182, 799–816.
Pilon, M., Cohu, C. M., Ravet, K., Abdel-Ghany, S. E., & Gaymard, F. (2009). Essential transition metal homeostasis in plants. Current Opinion in Plant Biology, 12, 347–357.
Puig, S., & Peñarrubia, L. (2009). Placing metal micronutrients in context: transport and distribution in plants. Current Opinion in Plant Biology, 12, 299–306.
Eisses, J. F., & Kaplan, J. H. (2002). Molecular characterization of hCTR1, the human copper uptake protein. Journal of Biological Chemistry, 277, 29162–29171.
Puig, S., Lee, J., Lau, M., & Thiele, D. J. (2002). Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. Journal of Biological Chemistry, 277, 26021–26030.
Vatansever, R., Ozyigit, I. I., & Filiz, E. (2016). Genome-wide identification and comparative analysis of copper transporter genes in plants. Interdisciplinary Sciences: Computational Life Sciences, 1–14.
Jiang, J., Nadas, I. A., Kim, M. A., & Franz, K. J. (2005). A Mets motif peptide found in copper transport proteins selectively binds Cu (I) with methionine-only coordination. Inorganic Chemistry, 44, 9787–9794.
Beaudoin, J., Laliberté, J., & Labbé, S. (2006). Functional dissection of Ctr4 and Ctr5 amino-terminal regions reveals motifs with redundant roles in copper transport. Microbiology, 152, 209–222.
Page, M. D., Kropat, J., Hamel, P. P., & Merchant, S. S. (2009). Two Chlamydomonas CTR copper transporters with a novel cys-met motif are localized to the plasma membrane and function in copper assimilation. Plant Cell Online, 21, 928–943.
Peñarrubia, L., Andrés-Colás, N., Moreno, J., & Puig, S. (2010). Regulation of copper transport in Arabidopsis thaliana: a biochemical oscillator? Journal of Biological Inorganic Chemistry, 15, 29–36.
Aller, S., Eng, G., De, E. T., Feo, C. J., & Unger, V. M. (2004). Eukaryotic CTR copper uptake transporters require two faces of the third transmembrane domain for helix packing, oligomerization, and function. Journal of Biological Chemistry, 279, 53435–53441.
Wu, X., Sinani, D., Kim, H., & Lee, J. (2009). Copper transport activity of yeast Ctr1 is down-regulated via its C terminus in response to excess copper. Journal of Biological Chemistry, 284, 4112–4122.
Abdel-Ghany, S. E., & Pilon, M. (2008). MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. Journal of Biological Chemistry, 283, 15932–15945.
Yamasaki, H., Hayashi, M., Fukazawa, M., Kobayashi, Y., & Shikanai, T. (2009). SQUAMOSA promoter binding protein–like7 is a central regulator for copper homeostasis in Arabidopsis. Plant Cell Online, 21, 347–361.
Kruse, T., Gehl, C., Geisler, M., Lehrke, M., Ringel, P., Hallier, S., et al. (2010). Identification and biochemical characterization of molybdenum cofactor-binding proteins from Arabidopsis thaliana. Journal of Biological Chemistry, 285, 6623–6635.
Mendel, R. R., & Leimkühler, S. (2015). The biosynthesis of the molybdenum cofactors. JBIC Journal of Biological Inorganic Chemistry, 20(2), 337–347.
Kaiser, B. N., Gridley, K. L., Brady, J. N., Phillips, T., & Tyerman, S. D. (2005). The role of molybdenum in agricultural plant production. Annual Botany, 96, 745–754.
Ide, Y., Kusano, M., Oikawa, A., Fukushima, A., Tomatsu, H., Saito, K., et al. (2011). Effects of molybdenum deficiency and defects in molybdate transporter MOT1 on transcript accumulation and nitrogen/Sulphur metabolism in Arabidopsis thaliana. Journal of Experimental Botany, 62, 1483–1497.
Hawkesford, M. J. (2003). Transporter gene families in plants: the sulphate transporter gene family-redundancy or specialization? Physiologia Plantarum, 117, 155–163.
Baxter, I., Muthukumar, B., Park, H. C., Buchner, P., Lahner, B., Danku, J., & Salt, D. E. (2008). Variation in molybdenum content across broadly distributed populations of Arabidopsis thaliana is controlled by a mitochondrial molybdenum transporter (MOT1). Plos Genetics, 4.
Bittner, F. (2014). Molybdenum metabolism in plants and crosstalk to iron. Frontier in Plant Sciences, 5.
Vatansever, R., Filiz, E., & Ozyigit, I. I. (2015). In silico identification and comparative analysis of molybdenum (Mo) transporter genes in plants. Brazilian Journal of Botany, 1–13.
Shibagaki, N., & Grossman, A. R. (2006). The role of the STAS domain in the function and biogenesis of a sulfate transporter as probed by random mutagenesis. Journal of Biological Chemistry, 281(32), 22964–22973.
Compton, E. L., Karinou, E., Naismith, J. H., Gabel, F., & Javelle, A. (2011). Low resolution structure of a bacterial SLC26 transporter reveals dimeric stoichiometry and mobile intracellular domains. Journal of Biological Chemistry, 286, 27058–27067.
Finn, R. D. (2012). Pfam: the protein families database. Encyclopedia of Genetics, Genomics, Proteomics and Bioinformatics.
Tejada-Jiménez, M., Llamas, Á., Sanz-Luque, E., Galván, A., & Fernández, E. (2007). A high- affinity molybdate transporter in eukaryotes. Proceedings of the National Academy of Sciences, 104, 20126–20130.
Zhu, Y., & Gong, H. (2014). Beneficial effects of silicon on salt and drought tolerance in plants. Agronomy for Sustainable Development, 34(2), 455–472.
Rodrigues, F. A., Resende, R. S., Dallagnol, L. J., & Datnoff, L. E. (2015). Silicon potentiates host defense mechanisms against infection by plant pathogens. In Silicon and Plant Diseases (pp. 109–138). Springer International Publishing.
Mitani, N., Yamaji, N., & Ma, J. F. (2008). Characterization of substrate specificity of a rice silicon transporter, Lsi1. Pflügers Archiv-European Journal of Physiology, 456(4), 679–686.
Zhang, C., Wang, L., Nie, Q., Zhang, W., & Zhang, F. (2008). Long-term effects of exogenous silicon on cadmium translocation and toxicity in rice (Oryza sativa L.). Environmental and Experimental Botany, 62, 300–307.
Ma, J. F. (2004). Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Science and Plant Nutrition, 50, 11–18.
Ashraf, M. A., Morshed, M. M., Ahammad, A. S., & Morshed, M. N. (2013). Computational study of silicon transporter protein in rice and wheat. International Journal of Computational Bioinformatics and In Silico Model, 2(4), 199–205.
Ma, J. F., Tamai, K., Yamaji, N., Mitani, N., Konishi, S., Katsuhara, M., et al. (2006). A silicon transporter in rice. Nature, 440, 688–691.
Bienert, G., & Chaumont, F. (2011). Plant aquaporins: roles in water homeostasis, nutrition, and signaling processes. In M. Geisler & K. Venema (Eds.), Transporters and pumps in plant signaling processes (pp. 3–36). Berlin: Springer-Verlag.
Wu, B., & Beitz, E. (2007). Aquaporins with selectivity for unconventional permeants. Cellular and Molecular Life Sciences, 64, 2413–2421.
Ilan, B., Tajkhorshid, E., Schulten, K., & Voth, G. A. (2004). The mechanism of proton exclusion in aquaporin channels. Proteins, 55, 223–228.
Forrest, K. L., & Bhave, M. (2007). Major intrinsic proteins (MIPs) in plants: a complex gene family with major impacts on plant phenotype. Functional Integrative Genomics, 7, 263–289.
Harries, W. E., Akhavan, D., Miercke, L. J., Khademi, S., & Stroud, R. M. (2004). The channel architecture of aquaporin 0 at a 2.2 A° resolution. Proceedings of the National Academy of Sciences, USA, 101, 14045–14050.
Mitani-Ueno, N., Yamaji, N., Zhao, F. J., & Ma, J. F. (2011). The aromatic/arginine selectivity filter of NIP aquaporins plays a critical role in substrate selectivity for silicon, boron, and arsenic. Journal of Experimental Botany, 62(12), 4391–4398.
Fu, D., Libson, A., Miercke, L. J., Weitzman, C., Nollert, P., Krucinski, J., et al. (2000). Structure of a glycerol-conducting channel and the basis for its selectivity. Science, 290, 481–486.
Sui, H., Han, B. G., Lee, J. K., Walian, P., & Jap, B. K. (2001). Structural basis of water-specific transport through the AQP1 water channel. Nature, 414, 872–878.
Ma, J. F., Yamaji, N., Mitani, N., Kazunori, T., Konishi, S., Fujiwara, T., et al. (2007). An efflux transporter of silicon in rice. Nature, 448, 209–212.
Ma, J. F. (2010). Si transporters in higher plant. In P. T. Jhon & P. G. Bienert (Eds.), MIPs and their role in the exchange of materials (pp. 99–109). Texas: Landes Bioscience.
Chiba, Y., Mitani, N., Yamaji, N., & Ma, J. F. (2009). HvLsi1 is a silicon influx transporter in barley. Plant Journal, 57, 810–818.
Mitani, N., Chiba, Y., Yamaji, N., & Ma, J. F. (2009). Identification and characterization of maize and barley Lsi2-like silicon efflux transporters reveals a distinct silicon uptake system from that in rice. Plant Cell, 21, 2133–2142.
Mitani, N., Yamaji, N., & Ma, J. F. (2009). Identification of maize silicon influx transporters. Plant and Cell Physiology, 50, 5–12.
Mitani, N., Yamaji, N., Ago, Y., Iwasaki, K., & Ma, J. F. (2011). Isolation and functional characterization of an influx silicon transporter in two pumpkin cultivars contrasting in silicon accumulation. Plant Journal, 66, 231–240.
Fernando, D. R., & Lynch, J. P. (2015). Manganese phytotoxicity: new light on an old problem. Annals of Botany, 116(3), 313–319.
Yin, C., Zhao, W., Zheng, L., Chen, L., Tan, Q., Shang, X., et al. (2014). High-level expression of a manganese superoxide dismutase (PoMn-SOD) from Pleurotus ostreatus in Pichia pastoris. Applied Biochemistry and Biotechnology, 174(1), 259–269.
Nickelsen, J., & Rengstl, B. (2013). Photosystem II assembly: from cyanobacteria to plants. Annual Review of Plant Biology, 64, 609–635.
Socha, A. L., & Guerinot, M. L. (2014). Mn-euvering manganese: the role of transporter gene family members in manganese uptake and mobilization in plants. Frontiers in Plant Sciences, 5, 106.
Cailliatte, R., Schikora, A., Briat, J. F., Mari, S., & Curie, C. (2010). High- affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. Plant Cell, 22, 904–917.
Vatansever, R., Filiz, E., & Ozyigit, I. I. (2016). In silico analysis of Mn transporters (NRAMP1) in various plant species. Molecular Biology Reports. doi:10.1007/s11033-016-3950-x.
Thomine, S., Lelievre, F., Debarbieux, E., Schroeder, J. I., & Barbier-Brygoo, H. (2003). AtNRAMP3, a multi specific vacuolar metal transporter involved in plant responses to iron deficiency. Plant Journal, 34, 685–695.
Lanquar, V., Ramos, M. S., Lelievre, F., Barbier-Brygoo, H., Krieger-Liszkay, A., Kramer, U., et al. (2010). Export of vacuolar manganese by AtNRAMP3 and AtNRAMP4 is required for optimal photosynthesis and growth under manganese deficiency. Plant Physiology, 152, 1986–1999.
Yamaji, N., Sasaki, A., Xia, J. X., Yokosho, K., & Ma, J. F. (2013). Anode-based switch for preferential distribution of manganese in rice. Nature Communications, 4, 2442.
Sasaki, A., Yamaji, N., Yokosho, K., & Ma, J. F. (2012). Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell Online, 24, 2155–2167.
Yang, T. J., Perry, P. J., Ciani, S., Pandian, S., & Schmidt, W. (2008). Manganese deficiency alters the patterning and development of root hairs in Arabidopsis. Journal of Experimental Botany, 59, 3453–3464.
Milner, M. J., Seamon, J., Craft, E., & Kochian, L. V. (2013). Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. Journal of Experimental Botany, 64, 369–381.
Koike, S., Inoue, H., Mizuno, D., Takahashi, M., Nakanishi, H., Mori, S., et al. (2004). OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant Journal, 39, 415–424.
Sasaki, A., Yamaji, N., Xia, J., & Ma, J. F. (2011). OsYSL6 is involved in the detoxification of excess manganese in rice. Plant Physiology, 157, 1832–1840.
Conte, S. S., Chu, H. H., Rodriguez, D. C., Punshon, T., Vasques, K. A., Salt, D. E., et al. (2013). Arabidopsis thaliana yellow stripe1-like4 and yellowstripe1-like6 localize to internal cellular membranes and are involved in metal ion homeostasis. Frontiers in Plant. Sciences, 4, 283.
Marshall, J., Corzo, A., Leigh, R. A., & Sanders, D. (1994). Membrane potential dependent calcium transport in right-side-out plasma membrane vesicles from Zea mays L. Roots. Plant Journal, 5, 683–694.
Wymer, C. L., Bibikova, T. N., & Gilroy, S. (1997). Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant Journal, 12, 427–439.
Curie, C., Alonso, J., Le Jean, M., Ecker, J., & Briat, J. (2000). Involvement of NRAMP1 from Arabidopsis thaliana in iron transport. Biochemical Journal, 347, 749–755.
Fabiano, C. C., Tezotto, T., Favarin, J. L., Polacco, J. C., & Mazzafera, P. (2015). Essentiality of nickel in plants: a role in plant stresses. Frontiers in plant science, 6.
Küpper, H., & Kroneck, P. M. (2007). Nickel in the environment and its role in the metabolism of plants and cyanobacteria. Metal Ions in Life Sciences, 2, 31–62.
Polacco, J. C., Mazzafera, P., & Tezotto, T. (2013). Opinion–nickel and urease in plants: still many knowledge gaps. Plant Science, 199, 79–90.
Maier, T., Jacobi, A., Sauter, M., & Böck, A. (1993). The product of the hypB gene, which is required for nickel incorporation into hydrogenases, is a novel guanine nucleotide-binding protein. Journal of Bacteriology, 175(3), 630–635.
van der Lelie, D., Wilmotte, A., & Wuertz, S. (1994). Plasmids for heavy metal resistance in Alcaligenes eutrophus CH34: mechanisms and applications. FEMS Microbiology Reviews, 14, 405–414.
Ragsdale, S. W. (1998). Nickel biochemistry. Current Opinion in Chemical Biology, 2(2), 208–215.
Mulrooney, S. B., & Hausinger, R. P. (2003). Nickel uptake and utilization by microorganisms. FEMS Microbiology Reviews, 27(2–3), 239–261.
Yusuf, M., Fariduddin, Q., Hayat, S., & Ahmad, A. (2011). Nickel: an overview of uptake, essentiality and toxicity in plants. Bulletin of Environmental Contamination and Toxicology, 86(1), 1–17.
Rajendran, P., Ashokkumar, B., Muthukrishnan, J., & Gunasekaran, P. (2002). Toxicity assessment of nickel using Aspergillus niger and its removal from an industrial effluent. Applied Biochemistry and Biotechnology, 102(1–6), 201–206.
Molas, J. (2002). Changes of chloroplast ultrastructure and total chlorophyll concentration in cabbage leaves caused by excess of organic Ni II complexes. Environmental and Experimental Botany, 47, 115–126.
Chen, C., Huang, D., & Liu, J. (2009). Functions and toxicity of nickel in plants: recent advances and future prospects. Clean, 37, 304–313.
Gajewska, E., Wielanek, M., Bergier, K., & Skłodowska, M. (2009). Nickel induced depression of nitrogen assimilation in wheat roots. Acta Physiologiae Plantarum, 31, 1291–1300.
Dan, T. V., Krishnaraj, S., & Saxena, P. K. (2002). Cadmium and nickel uptake and accumulation in scented geranium (Pelargonielm sp. ‘Frensham’). Water. Air and Soil Pollution, 137, 355–364.
Vogel-Mikus, K., Drobne, D., & Regvar, M. (2005). Zn, Cd and Pb accumulation and arbuscular mycorrhizal colonization of pennycress Thlaspi Praecox Wulf. (Brassicaceae) from the vicinity of a lead mine and smelter in Slovenia. Environmental Pollution, 133, 233–242.
Seregin, I. V., & Kozhevnikova, A. D. (2006). Physiological role of nickel and its toxic effects on higher plants. Russian Journal of Plant Physiology, 53, 257–277.
Nishida, S., Tsuzuki, C., Kato, A., Aisu, A., Yoshida, J., & Mizuno, T. (2011). AtIRT1, the primary iron uptake transporter in the root, mediates excess nickel accumulation in Arabidopsis thaliana. Plant and Cell Physiology, 52(8), 1433–1442.
Baetz, U., Eisenach, C., Tohge, T., Martinoia, E., & De Angeli, A. (2016). Vacuolar chloride fluxes impact ion content and distribution during early salinity stress. Plant Physiology, 00183.
Teodoro, A. E., Zingarelli, L., & Lado, P. (1998). Early changes of Cl efflux band H+ extrusion induced by osmotic stress in Arabidopsis thaliana cells. Physiologia Plantarum, 102, 29–37.
Xu, G., Magen, H., Tarchitzky, J., & Kafkafi, U. (2000). Advances in chloride nutrition. Advances in Agronomy, 68, 96–150.
White, P. J., & Broadley, M. R. (2001). Chloride in soils and its uptake and movement within the plant: a review. Annals of Botany, 88, 967–988.
Teakle, N. L., & Tyerman, S. D. (2010). Mechanisms of Cl-transport contributing to salt tolerance. Plant, Cell & Environment, 33(4), 566–589.
Moya, J. L., Gomez-Cadenas, A., Primo-Millo, E., & Talon, M. (2003). Chloride absorption in salt-sensitive Carrizo citrange and salt tolerant Cleopatra mandarin citrus rootstocks is linked to water use. Journal of Experimental Botany, 54, 825–833.
Franklin, J. A., & Zwiazek, J. J. (2004). Ion uptake in Pinus banksiana treated with sodium chloride and sodium sulphate. Physiologia Plantarum, 120, 482–490.
Luo, Q., Bingjun, Y., & Liu, Y. (2005). Differential selectivity to chloride and sodium ions in seedlings of Glycine max and G. soja under NaCl stress. Journal of Plant Physiology, 162, 1003–1012.
Teakle, N. L., Real, D., & Colmer, T. D. (2006). Growth and ion relations in response to combined salinity and waterlogging in the perennial forage legumes Lotus corniculatus and Lotus tenuis. Plant and Soil, 289, 369–383.
Teakle, N., Flowers, T., Real, D., & Colmer, T. (2007). Lotus tenuis Tolerates the interactive effects of salinity and waterlogging by ‘excluding’ Na+ and Cl- from the xylem. Journal of Experimental Botany, 58, 2169–2180.
Pilon-Smits, E. A. (2015). Selenium in plants. In Progress in botany (pp. 93–107). Springer International Publishing.
Chu, J. Z., Yao, X. Q., & Zhang, Z. N. (2010). Responses of wheat seedlings to exogenous selenium supply under cold stress. Biological Trace Element Research, 136(3), 355–363.
Hasanuzzaman, M., & Fujita, M. (2011). Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biological Trace Element Research, 143, 1758–1776.
Djanaguiraman, M., Prasad, P. V. V., & Seppänen, M. (2010). Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiology and Biochemistry, 48(12), 999–1007.
Pukacka, S., Ratajczak, E., & Kalemba, E. (2011). The protective role of selenium in recalcitrant Acer saccharium L. Seeds subjected to desiccation. Journal of Plant Physiology, 168(3), 220–225.
Kumar, M., Bijo, A. J., Baghel, R. S., Reddy, C. R. K., & Jha, B. (2012). Selenium and Spermine alleviates cadmium induced toxicity in the red seaweed Gracilaria dura by regulating antioxidant system and DNA methylation. Plant Physiology and Biochemistry, 51, 129–138.
Wang, C. Q. (2011). Water-stress mitigation by selenium in Trifolium repens L. Journal of Plant Nutrition and Soil Science, 174(2), 276–282.
Hartikainen, H., Xue, T. L., & Piironen, V. (2000). Selenium as an anti-oxidant and prooxidant in ryegrass. Plant and Soil, 225, 193–200.
Yao, X., Chu, J., & Ba, C. (2010). Antioxidant responses of wheat seedlings to exogenous selenium supply under enhanced ultraviolet-B. Biological Trace Element Research, 136(1), 96–105.
Pezzarossa, B., Remorini, D., Gentile, M. L., & Massai, R. (2012). Effects of foliar and fruit addition of sodium selenate on selenium accumulation and fruit quality. Journal of the Science of Food and Agriculture, 92, 781–786.
Belokobylsky, A., Ginturi, E., Kuchava, N., Kirkesali, E., Mosulishvili, L., Frontasyeva, M., et al. (2004). Accumulation of selenium and chromium in the growth dynamics of Spirulina platensis. Journal of Radioanalytical and Nuclear Chemistry, 259(1), 65–68.
Feng, R. W., & Wei, C. Y. (2012). Antioxidative mechanisms on selenium accumulation in Pteris vittata L., a potential selenium phytoremediation plant. Plant, Soil and Environment, 58(3), 105–110.
Fordyce, F.M. (2005). Selenium deficiency and toxicity in the environment. In Essentials of Medical Geology (Selinus, O. et al., eds), Elsevier pp. 373–415.
Elrashidi, M. A., et al. (1987). Chemical-equilibria of seleniumin soils—a theoretical development. Soil Science, 144, 141–152.
Anderson, J. (1993). Selenium interactions in sulfur metabolism. In Sulfur nutrition and assimilation in higher plants: regulatory agricultural and environmental aspects (De Kok, I.S. et al., eds), SPB Academic, pp. 49–60.
White, P. J., Bowen, H. C., Parmaguru, P., Fritz, M., Spracklen, W. P., Spiby, R. E., et al. (2004). Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. Journal of Experimental Botany, 55(404), 1927–1937.
Zhu, Y. G., Pilon-Smits, E. A., Zhao, F. J., Williams, P. N., & Meharg, A. A. (2009). Selenium in higher plants: understanding mechanisms for biofortification and phytoremediation. Trends in Plant Science, 14(8), 436–442.
Arvy, M. P. (1993). Selenate and selenite uptake and translocation in bean-plants (Phaseolus vulgaris. Journal of Experimental Botany, 44, 1083–1087.
Li, H. F., McGrath, S. P., & Zhao, F. J. (2008). Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytologist, 178(1), 92–102.
Zhang, J. L., Flowers, T. J., & Wang, S. M. (2010). Mechanisms of sodium uptake by roots of higher plants. Plant and Soil, 326(1–2), 45–60.
Flowers, T. J., & Yeo, A. R. (1988). Ion relation of salt tolerance. In: Baker DA, Hall JL (eds) Solute transport in plant cells and tissues. Longman Scientific and Technical, Harlow, pp 392–413.
Tester, M., & Davenport, R. (2003). Na+ tolerance and Na+ transport in higher plants. Annual Botany, 91, 503–527.
Flowers, T. J., & Colmer, T. D. (2008). Salinity tolerance in halophytes. New Phytologist, 179, 945–963.
Kronzucker, H. J., & Britto, D. T. (2011). Sodium transport in plants: a critical review. New Phytologist, 189(1), 54–81.
Amtmann, A., & Sanders, D. (1999). Mechanism of Na+ uptake by plant cells. Advances in Botanical Research, 29, 75–112.
Tyerman, S. D., & Skerrett, I. M. (1999). Root ion channels and salinity. Scientia Horticulturae, 78, 175–235.
White, P. J. (1999). The molecular mechanism of sodium influx to root cells. Trends in Plant Science, 4(7), 245–246.
Antosiewicz, D. M., & Hennig, J. (2004). Overexpression of LCT1 in tobacco enhances the protective action of calcium against cadmium toxicity. Environmental Pollution, 129, 237–245.
Liu, W. H., Schachtman, D. P., & Zhang, W. (2000). Partial deletion of a loop region in the high affinity K+ transporter HKT1 changes ionic permeability leading to increased salt tolerance. Journal of Biological Chemistry, 275, 27924–27932.
Blumwald, E., Aharon, G. S., & Apse, M. P. (2000). Sodium transport in plant cells. Biochim Biophys Acta-Biomembranes, 1465, 140–151.
?>Senn, M. E., Rubio, F., Banuelos, M. A., & Rodrıguez-Navarro, A. (2001). Comparative functional features of plant potassium HvHAK1 and HvHAK2 transporters. Journal of Biological Chemistry, 30, 44563–44569.
Colmenero-Flores, J. M., Martinez, G., Gamba, G., Vazquez, N., Iglesias, D. J., Brumos, J., et al. (2007). Identification and functional characterization of cation-chloride cotransporters in plants. Plant Journal, 50, 278–292.
Hajiboland, R., Bahrami Rad, S., Barceló, J., & Poschenrieder, C. (2013). Mechanisms of aluminum-induced growth stimulation in tea (Camellia sinensis). Journal of Plant Nutrition and Soil Science, 176(4), 616–625.
Barcelo, J., & Poschenrieder, C. (2002). Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: a review. Environmental and Experimental Botany, 48(1), 75–92.
Sade, H., Meriga, B., Surapu, V., Gadi, J., Sunita, M. S. L., Suravajhala, P., et al. (2016). Toxicity and tolerance of aluminum in plants: tailoring plants to suit to acid soils. Biometals, 29(2), 187–210.
Poschenrieder, C., Gunsé, B., Corrales, I., & Barceló, J. (2008). A glance into aluminum toxicity and resistance in plants. Science of the Total Environment, 400(1), 356–368.
Konishi, S. (1992). Promotive effects of aluminium on tea plant growth. Japan Agricultural. Research Quarterly, 26, 26–26.
Watanabe, T., & Osaki, M. (2002). Mechanisms of adaptation to high aluminum condition in native plant species growing in acid soils: a review. Communications in Soil Sciences and Plant Analysis, 33, 1247–1260.
Jansen, S., Watanabe, T., Caris, P., Geuten, K., Lens, F., Pyck, N., et al. (2004). The distribution and phylogeny of aluminium accumulating plants in the Ericales. Plant Biology, 6, 498–505.
Osawa, H., Ikeda, S., & Tange, T. (2013). The rapid accumulation of aluminum is ubiquitous in both the evergreen and deciduous leaves of Theaceae and Ternstroemiaceae plants over a wide pH range in acidic soils. Plant and Soil, 363(1–2), 49–59.
Hajiboland, R., Barceló, J., Poschenrieder, C., & Tolrà, R. (2013). Amelioration of iron toxicity: a mechanism for aluminum-induced growth stimulation in tea plants. Journal of Inorganic Biochemistry, 128, 183–187.
Kinraide, T. B. (1994). Use of a Gouy-Chapman-stern model for membrane-surface electrical potential to interpret some features of mineral rhizotoxicity. Plant Physiology, 106(4), 1583–1592.
Kidd, P. S., & Proctor, J. (2001). Why plants grow poorly on very acid soils: are ecologists missing the obvious? Journal of Experimental Botany, 52(357), 791–799.
Ghanati, F., Morita, A., & Yokota, H. (2005). Effects of aluminum on the growth of tea plant and activation of antioxidant system. Plant and Soil, 276(1–2), 133–141.
Komeda, H., Kobayashi, M., & Shimizu, S. (1997). A novel transporter involved in cobalt uptake. Proceedings of the National Academy of Sciences, 94, 36–41.
Bakkaus, E., Gouget, B., Gallien, J. P., Khodja, H., Carrot, F., Morel, J. L., et al. (2005). Concentration and distribution of cobalt in higher plants: the use of micro-PIXE spectroscopy. Nuclear Instruments and Methods in Physic Research, 231, 350.
Sree, K. S., Keresztes, Á., Mueller-Roeber, B., Brandt, R., Eberius, M., Fischer, W., et al. (2015). Phytotoxicity of cobalt ions on the duckweed Lemna minor—morphology, ion uptake, and starch accumulation. Chemosphere, 131, 149–156.
Cai, Z., Kastell, A., Speiser, C., & Smetanska, I. (2013). Enhanced resveratrol production in Vitis vinifera cell suspension cultures by heavy metals without loss of cell viability. Applied Biochemistry and Biotechnology, 171(2), 330–340.
Jayakumar, K., Jaleel, C. A., & Azooz, M. M. (2008). Impact of cobalt on germination and seedling growth of Eleusine coracana L. And Oryza sativa L. Under hydroponic culture. Global Journal of Molecular Sciences, 3(1), 18–20.
Wei, W., Wang, Y., Wei, Z. G., Zhao, H. Y., Li, H. X., & Hu, F. (2009). Roles of organic acids and nitrate in the long-distance transport of cobalt in xylem saps of Alyssum murale and Trifolium subterraneum. Biological Trace Element Research, 131(2), 165–176.
Karovic, O., Tonazzini, I., Rebola, N., Edström, E., Lövdahl, C., Fredholm, B. B., et al. (2007). Toxic effects of cobalt in primary cultures of mouse astrocytes similarities with hypoxia and role of HIF-1α. Biochemical Pharmacology, 73, 694–708.
Palit, S., Sharma, A., & Talukder, G. (1994). Effects of cobalt on plants. Botanical Review, 60, 149–181.
Barysas, D., Cesniene, T., Balciuniene, L., Vaitkuniene, V., & Rancelis, V. (2002). Genotoxicity of Co2+ in plants and other organisms. Biologija, 2, 58–63.
Kukier, U., Peters, C. A., Chaney, R. L., Angle, J. S., & Roseberg, R. J. (2004). The effect of pH on metal accumulation in two alyssum species. Journal of Environmental Quality, 33, 2090–2102.
Li, Z., McLaren, R. G., & Metherell, A. K. (2004). The availability of native and applied soil cobalt to ryegrass in relation to soil cobalt and manganese status and other soil properties. New Zealand Journal of Agricultural Research, 47, 33–43.
Li, H. F., Gray, C., Mico, C., Zhao, F. J., & McGrath, S. P. (2009). Phytotoxicity and bioavailability of cobalt to plants in a range of soils. Chemosphere, 75(7), 979–986.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vatansever, R., Ozyigit, I.I. & Filiz, E. Essential and Beneficial Trace Elements in Plants, and Their Transport in Roots: a Review. Appl Biochem Biotechnol 181, 464–482 (2017). https://doi.org/10.1007/s12010-016-2224-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2224-3