Abstract
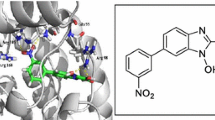
Lactate dehydrogenase C4 (LDH-C4) is considered to be a target protein for the development of contraceptives. In this work, the characterization of plateau zokor LDH-C4 and the screening of a series of N-substituted oxamic acids as inhibitors against zokor LDH-C4 were reported. The cDNA of zokor LDH-C gene was cloned and expressed in Escherichia coli, from which the protein was purified and further characterized. The protein was a tetramer (LDH-C4) and thermally stable up to 62 °C with a K m of 63.9 μM for pyruvate and with optimal pH values of 7.95 and 10.1 for the forward and backward reactions respectively. Virtual and in vitro screening against zokor LDH-C4 revealed eight N-substituted oxamic acids with IC50s ranging from 198 to 2513 μM, higher than that of oxamic acid (150 μM) and (ethylamino)(oxo)acetic acid (59 μM). The inhibition potencies of N-substituted oxamic acids tested are in the micromolar range, and the increase in the length of substituting chain seems not to increase inhibition potency.


Similar content being viewed by others
References
Wei, D. B., Wei, L., Zhang, J. M., & Yu, H. Y. (2006). Blood-gas properties of plateau zokor (Myospalax baileyi). Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology, 145, 372–375.
Su, J. H., Liu, R. T., Ji, W. H., Jiao, T., Cai, Z. S., & Hua, L. M. (2013). Stages and characteristics of grassland rodent pests control and research in China. Pratacultural Science, 30, 1116–1123 (in Chinese).
Goldberg, E. (1985). Reproductive implications of LDH-C4 and other testis-specific isozymes. Experimental and Clinical Immunogenetics, 2, 120–124.
Markert, C. L., Shaklee, J. B., & Whitt, G. S. (1975). Evolution of a gene. Multiple genes for LDH isozymes provide a model of the evolution of gene structure, function and regulation. Science, 189, 102–114.
Tsuji, S., Qureshi, M. A., Hou, E. W., Fitch, W. M., & Li, S. S. (1994). Evolutionary relationships of lactate dehydrogenases (LDHs) from mammals, birds, an amphibian, fish, barley, and bacteria: LDH cDNA sequences from Xenopus, pig, and rat. Proceedings of the National Academy of Sciences of the United States of America, 91, 9392–9396.
Goldberg, E. (1987). Antigenic sites of lactate dehydrogenase-C4. Isozymes: Current Topics Biological Medical Research, 14, 103–122.
O’hern, P. A., Bambra, C. S., Isahakia, M., & Goldberg, E. (1995). Reversible contraception in female baboons immunized with a synthetic epitope of sperm-specific lactate dehydrogenase. Biology of Reproduction, 52, 331–339.
Shi, S. Q., Wang, J. L., Peng, J. P., Chang, J. J., & Yang, Y. (2005). Oral feeding and nasal instillation immunization with Microtus brandti lactate dehydrogenase C epitope DNA vaccine reduces fertility in mice via specific antibody responses. Fertility and Sterility, 84, 781–784.
Odet, F., Duan, C., Willis, W. D., Goulding, E. H., Kung, A., Eddy, E. M., & Goldberg, E. (2008). Expression of the gene for mouse lactate dehydrogenase C (Ldhc) is required for male fertility. Biology of Reproduction, 79, 26–34.
Blanco, A., Burgos, C., Gerez de Burgos, N. M., & Montamat, E. E. (1976). Properties of the testicular lactate dehydrogenase isoenzyme. The Biochemical Journal, 153, 165–172.
Rodríguez-Páez, L., Chena-Taboada, M. A., Cabrera-Hernández, A., Cordero-Martínez, J., & Wong, C. (2011). Oxamic acid analogues as LDH-C4-specific competitive inhibitors. Journal of Enzyme Inhibition and Medicinal Chemistry, 26, 579–586.
Yu, Y., Deck, J. A., Hunsaker, L. A., Deck, L. M., Royer, R. E., Goldberg, E., & Vander Jagt, D. L. (2001). Selective active site inhibitors of human lactate dehydrogenases A4, B4, and C4. Biochemical Pharmacology, 62, 81–89.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Dietz, A. A., & Lubrano, T. (1967). Separation and quantitation of lactic dehydrogenase isoenzymes by disc electrophoresis. Analytical Biochemistry, 20, 246–257.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Bordoli, L., Kiefer, F., Arnold, K., Benkert, P., Battey, J., & Schwede, T. (2009). Protein structure homology modeling using SWISS-MODEL workspace. Nature Protocols, 2009(4), 1–13.
Benkert, P., Biasini, M., & Schwede, T. (2011). Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics, 27, 343–350.
Cosconati, S., Forli, S., Perryman, A. L., Harris, R., Goodsell, D. S., & Olson, A. J. (2010). Virtual screening with AutoDock: theory and practice. Expert Opinion on Drug Discovery, 5, 597–607.
Kolb, E., Fleisher, G. A., & Larner, J. (1970). Isolation and characterization of bovine lactate dehydrogenase X. Biochemistry, 9, 4372–4380.
Lee, C. Y., Yuan, J. H., & Goldberg, E. (1982). Lactate dehydrogenase isozymes from mouse. Methods in Enzymology, 89, 351–358.
LeVan, K. M., & Goldberg, E. (1991). Properties of human testis-specific lactate dehydrogenase expressed from Escherichia coli. The Biochemical Journal, 273, 587–592.
Battellino, L. J., Jaime, F. R., & Blanco, A. (1968). Kinetic properties of rabbit testicular lactate dehydrogenase isozyme. The Journal of Biological Chemistry, 243, 5185–5192.
Schatz, L., & Segal, H. L. (1969). Reduction of alpha-ketoglutarate by homogeneous lactic dehydrogenase X of testicular tissue. The Journal of Biological Chemistry, 244, 4393–4397.
Acknowledgments
We thank Dr. Min Yang for the language editing of this manuscript. Funds were provided by the National Science Foundation of China (31071700); the Youth Foundation of Sichuan province (2015JQO049); the scientific research fund of Sichuan Provincial Science and Technology Department (2015JY0232); Sichuan Provincial Health Department (130310); and the Fundamental Research Funds for the Central Universities, Southwest University for Nationalities (2015NYB13).
Author information
Authors and Affiliations
Corresponding author
Additional information
Qinghua He and Qinglian Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
He, Q., Zhang, Q., Huang, L. et al. Characterization and Inhibitor Screening of Plateau Zokor Lactate Dehydrogenase C4. Appl Biochem Biotechnol 179, 927–937 (2016). https://doi.org/10.1007/s12010-016-2040-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2040-9