Abstract
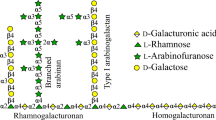
Galactanases (endo-β-1,4-galactanases—EC 3.2.1.89) catalyze the hydrolysis of β-1,4 galactosidic bonds in arabinogalactan and galactan side chains found in type I rhamnogalacturan. The aim of this work was to understand the catalytic function, biophysical properties, and use of a recombinant GH53 endo-beta-1,4-galactanase for commercial cocktail supplementation. The nucleotide sequence of the endo-β-1,4-galactanase from Bacillus licheniformis CBMAI 1609 (Bl1609Gal) was cloned and expressed in Escherichia coli, and the biochemical and biophysical properties of the enzyme were characterized. The optimum pH range and temperature of Bl1609Gal activity were 6.5–8 and 40 °C, respectively. Furthermore, Bl1609Gal showed remarkable pH stability, retaining more than 75 % activity even after 24 h of incubation at pH 4–10. The enzyme was thermostable, retaining nearly 100 % activity after 1-h incubation at pH 7.0 at 25–45 °C. The enzymatic efficiency (K cat /K m ) against potato galactan under optimum conditions was 241.2 s−1 mg−1 mL. Capillary zone electrophoresis demonstrated that the pattern of galactan hydrolysis by Bl1609Gal was consistent with that of endogalactanases. Supplementation of the commercial cocktail ACCELLERASE®1500 with recombinant Bl1609Gal increased hydrolysis of pretreated sugarcane bagasse by 25 %.





Similar content being viewed by others
References
Nanda, S., Mohammad, J., Reddy, S. N., Kozinski, J. A., & Dalai, A. K. (2014). Pathways of lignocellulosic biomass conversion to renewable fuels. Biomass Conversion and Biorefinery, 4, 157–91.
Mathews, S. L., Pawlak, J., & Grunden, A. M. (2015). Bacterial biodegradation and bioconversion of industrial lignocellulosic streams. Applied Microbiology and Biotechnology, 99, 2939–54.
Howard, R. L., Abotsi, E., Jansen van Rensburg, E. L., & Howard, S. (2003). Lignocellulose biotechnology: issues of bioconversion and enzyme production. African Journal of Biotechnology, 2(12), 602–19.
Singhvi, M. S., Chaudhari, S., & Gokhale, D. V. (2014). Lignocellulose processing: a current challenge. RSC Advances, 4, 8271–7.
Bozell, J. J., & Petersen, G. R. (2010). Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chemistry, 12(4), 539–54.
Tu, T., Bai, Y., Luo, H., Ma, R., Wang, Y., Shi, P., Yang, P., Meng, K., & Yao, B. (2014). A novel bifunctional pectinase from Penicillium oxalicum SX6 with separate pectin methylesterase and polygalacturonase catalytic domains. Applied Microbiology and Biotechnology, 98, 5019–28.
Willats, W. G. T., McCartney, L., Mackie, W., & Knox, J. P. (2001). Pectin: cell biology and prospects for functional analysis. Plant Molecular Biology, 47, 9–27.
Caffall, K. H., & Mohnen, D. (2009). The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydrate Research, 344, 1879–900.
Sharma, N., Rathore, M., & Sharma, M. (2013). Microbial pectinase: sources, characterization and application. Reviews in Environmental Science and Bio/Technology, 12, 45–60.
Torpenholt, S., Le Nours, J., Christensen, U., Jahn, M., Withers, S., Ostergaard, P. R., Borchert, T. V., Pousen, J. C., & Lo Leggio, L. (2011). Activity of three β-1,4-galactanases on small chromogenic substrates. Carbohydrate Research, 346, 2028–33.
Le Nours, J., De Maria, L., Welner, D., Jorgensen, C. T., Christensen, L. L. H., Borchert, T. V., Larsen, S., & Lo Leggio, L. (2008). Investigating the binding of β-1,4-galactan to Bacillus licheniformis β-1,4-galactanase by crystallography and computational modeling. Proteins, 75(4), 977–89.
Bjornvad, M. E., Clausen, I. G., Schulein, M., Bech, L., Ostergaard, P. R., & Sjoholm, C. (2001). Bacterial galactanases and use thereof. United States Patent, 6, 331,426.
Souza, A. P., Leite, D. C. C., Pattathil, S., Hahn, M. G., & Buckeridge, M. S. (2013). Composition and structure of sugarcane cell wall polysaccharides: implications for second-generation bioethanol production. BioEnergy Research, 6(2), 564–79.
Pandey, A., Soccol, C. R., Nigam, P., & Soccol, V. T. (2000). Biotechnological potential of agro-industrial residues, I: sugarcane bagasse. Bioresource Technology, 74(1), 69–80.
Li, J., Zhou, P., Liu, H., Xiong, C., Lin, J., Xiao, W., Gong, Y., & Liu, Z. (2014). Synergism of cellulase, xylanase, and pectinase on hydrolyzing sugarcane bagasse resulting from different pretreatment technologies. Bioresource Technology, 155, 258–65.
Berlin, A., Maximenko, V., Gilkes, N., & Saddler, J. (2007). Optimization of enzyme complexes for lignocellulose hydrolysis. Biotechnology and Bioengineering, 97(2), 287–96.
Delabona, P. D. S., Cota, J., Hoffmam, Z. B., Paixão, D. A. A., Farinas, C. S., Cairo, J. P. L. F., Lima, D. J., Squina, F. M., Ruller, R., & Pradella, J. G. C. (2013). Understanding the cellulolytic system of Trichoderma harzianum P49P11 and enhancing saccharification of pretreated sugarcane bagasse by supplementation with pectinase and α-L-arabinofuranosidase. Bioresource Technology, 131, 500–7.
Machado, C. B., Citadini, A. P., Goldbeck, R., de Lima, E. A., Figueiredo, F. L., da Silva, T. M., Hoffmam, Z. B., de Sousa, A. S., Squina, F. M., Polizeli, M. L. T. M., Ruller, R., & Ward, R. J. (2015). Increased biomass saccharification by supplementation of a commercial enzyme cocktail with endo-arabinanase from Bacillus licheniformis. Biotechnology Letters, 37(7), 1455–62.
Zhang, J., Pakarinen, A., & Viikari, L. (2013). Synergy between cellulases and pectinases in the hydrolysis of hemp. Bioresource Technology, 129, 302–7.
Alvarez, T. M., Goldbeck, R., Dos Santos, C. R., Paixão, D. A., Gonçalves, T. A., Cairo, J. P. L. F., Almeida, R. F., Pereira, I. O., Jackson, G., Cota, J., Büchli, F., Citadini, A. P., Ruller, R., Polo, C. C., Neto, M. O., Murakami, M. T., & Squina, F. M. (2013). Development and biotechnological application of a novel endoxylanase family GH10 identified from sugarcane soil metagenome. Plos One, 8(7), e70014. doi:10.1371/journal.pone.0070014.
Miller, G. L. (1959). Use dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426–8.
Evangelista, R. A., Liu, M. S., & Chen, F. T. A. (1995). Characterization of 9-aminopyrene-1,4,6-trisulfonate derivatized sugars by capillary electrophoresis with laser-induced fluorescence detection. Analytical Chemistry, 67(13), 2239–45.
Cota, J., Alvarez, T. M., Citadini, A. P., Santos, C. R., de Oliveira Neto, M., Oliveira, R. R., Pastore, G. M., Ruller, R., Prade, R. A., Murakami, M. T., & Squina, F. M. (2011). Mode of operation and low-resolution structure of a multi-domain and hyperthermophilic endo-β-1,3-glucanase from Thermotoga petrophila. Biochemical Biophysical Research Communications, 406, 590–4.
Bragatto, J., Segato, F., & Squina, F. M. (2013). Production of xylooligosaccharides (XOS) from delignified sugarcane bagasse by peroxide-HAc process using recombinant xylanase from Bacillus subtilis. Industrial Crops and Products, 51, 123–9.
Tsumura, K., Hashimoto, Y., Akiba, T., & Horikoshi, K. (1991). Purification and properties of galactanases from alkalophilic Bacillus spS-2 and S-39. Agricultural and Biological Chemistry, 55(5), 1265–71.
Corrêa, D. H. A., & Ramos, C. H. I. (2009). The use of circular dichroism spectroscopy to study protein folding, form and function. African Journal of Biochemistry Research, 3(5), 164–73.
Ryttersgaard, C., Nours, J. L., Leggio, L. L., Jorgensen, C. T., Christensen, L. L. H., Bjornvad, M., & Larsen, S. (2004). The structure of endo-β-1,4-galactanase from Bacillus licheniformis in complex with two oligosaccharide products. Journal of Molecular Biology, 341(1), 107–17.
Emi, S., & Yamoto, T. (1972). Purification and properties of several galactanases of Bacillus subtilis var. amylosacchariticus. Agricultural and Biological Chemistry, 36(11), 1945–54.
Sakamoto, T., & Ishimaru, M. (2013). Peculiarities and applications of galactanolytic enzymes that act on type I and II arabinogalactans. Applied Microbiology and Biotechnology, 97(12), 5201–13.
Yamamoto, T., & Emi, S. (1998). Arabinogalactanase of Bacillus subtilis var. amylosacchariticus. Methods in Enzymology, 160, 719–25.
Tabachnikov, O., & Shoham, Y. (2013). Functional characterization of the galactan utilization system of Geobacillus stearothermophilus. The FEBS Journal, 280, 950–64.
Braithwaite, K. L., Barna, T., Spurway, T. D., Charnock, S. J., Black, G. W., Hughes, N., Lakey, J. H., Virden, R., Hazlewood, G. P., Henrissat, B., & Gilbert, H. J. (1997). Evidence that galactanase A from Pseudomonas fluorescens subspecies cellulosa is a retaining family 53 glycosyl hydrolase in which E161 and E270 are the catalytic residues. Biochemistry, 36(49), 15489–500.
Banerjee, G., Scott-Craig, J. S., & Walton, J. D. (2010). Improving enzymes for biomass conversion: a basic research perspective. BioEnergy Research, 3, 82–92.
Banerjee, G., Car, S., Scott-Craig, J. S., Borrusch, M. S., Bongers, M., & Walton, J. D. (2010). Synthetic multi-component enzyme mixtures for deconstruction of lignocellulosic biomass. Bioresource Technology, 101, 9097–105.
Acknowledgments
This work was supported by CNPq (481666/2012-5), CAPES, and FAPESP (2013/14454-3) research grants. We also thank the Laboratório de Análises de Macromoléculas (LAM) at Centro Nacional de Pesquisa em Energia e Materiais (CNPEM) for their support on spectroscopic experiments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM. 1
(DOCX 234 kb)
Rights and permissions
About this article
Cite this article
de Lima, E.A., Machado, C.B., Zanphorlin, L.M. et al. GH53 Endo-Beta-1,4-Galactanase from a Newly Isolated Bacillus licheniformis CBMAI 1609 as an Enzymatic Cocktail Supplement for Biomass Saccharification. Appl Biochem Biotechnol 179, 415–426 (2016). https://doi.org/10.1007/s12010-016-2003-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2003-1