Abstract
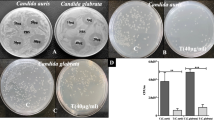
Allium sativum is well known for its medicinal properties. The A. sativum lectin 50 (ASL50, 50 kDa) was isolated from aged A. sativum bulbs and purified by gel filtration chromatography on Sephacryl S-200 column. Agar well diffusion assay were used to evaluate the antimicrobial activity of ASL50 against Candida species and bacteria then minimal inhibitory concentration (MIC) was determined. The lipid A binding to ASL50 was determined by surface plasmon resonance (SPR) technology with varying concentrations. Electron microscopic studies were done to see the mode of action of ASL50 on microbes. It exerted antimicrobial activity against clinical Candida isolates with a MIC of 10–40 μg/ml and clinical Pseudomonas aeruginosa isolates with a MIC of 10–80 μg/ml. The electron microscopic study illustrates that it disrupts the cell membrane of the bacteria and cell wall of fungi. It exhibited antiproliferative activity on oral carcinoma KB cells with an IC50 of 36 μg/ml after treatment for 48 h and induces the apoptosis of cancer cells by inducing 2.5-fold higher caspase enzyme activity than untreated cells. However, it has no cytotoxic effects towards HEK 293 cells as well as human erythrocytes even at higher concentration of ASL50. Biological properties of ASL50 may have its therapeutic significance in aiding infection and cancer treatments.






Similar content being viewed by others
Abbreviations
- LA:
-
Lipid A
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- MHA:
-
Mueller Hinton agar
- SDA:
-
Sabouraud dextrose agar
- MIC:
-
Minimal inhibitory concentration
- CLSI:
-
Clinical and Laboratory Standards Institute
- MHB:
-
Mueller Hinton broth media
- TEM:
-
Transmission electron microscopy
- SEM:
-
Scanning electron microscopy
References
Palaksha M. N., Ahmed M., & Das S. (2010). Antibacterial activity of garlic extracts on streptomycin-resistant Staphylococcus aurous and Escherichia coli solely and in synergism with streptomycin. Journal of Natural Science, Biology and Medicine, 1, 12–15.
Fujisawa H., Watanabe K., Suma K., Origuchi K., Matsufuji H., Seki T., & Ariga T. (2009). Antibacterial potential of garlic-derived allicin and its cancellation by sulfhydryl compounds. Bioscience, Biotechnology, and Biochemistry, 73, 1948–1955.
Holzgartner H., Schmidt U., & Kuhn U. (1992). Congress of medical plant. European Journal of Medical Research, 3, 8–9.
Thomson M., & Ali M. (2003). A review of its potential use as an anti-cancer agent. Current Cancer Drug Targets, 3, 67–81.
Wang H. X., & Ng T. B. (2001). Purification of allivin, a novel antifungal protein from bulbs of the round-cloved garlic. Life Sciences, 70, 357–365.
Xia L., & Ng T. B. (2005). Isolation of alliumin, a novel protein with antimicrobial and antiproliferative activities from multiple-cloved garlic bulbs. Peptides, 26, 177–183.
Dam T. K., Bachhawat K., Rani P. G., & Surolia A. (1998). Garlic (Allium sativum) lectins bind to high mannose oligosaccharide chains. Journal of Biological Chemistry, 273, 5528–5535.
Gupta, A., & Sandhu, R.S. (1997). A new high molecular weight agglutinin from garlic (Allium sativum). Molecular and Cellular Biochemistry, 166 (1-2), 1-9.
Chandra N. R., Ramachandraiah G., Bachhawat K., Dam T. K., Surolia A., & Vijayan M. (1999). Crystal structure of a dimeric mannose-specific agglutinin from garlic: quaternary association and carbohydrate specificity. Journal of Biological Chemistry, 285(3), 1157–1168.
Laemmli U. K., & Favre M. (1973). Maturation of the head of bacteriophage T4. I. DNA packaging events. Journal of Biological Chemistry, 80, 575–599.
Perez C., & Anesini C. (1994). In vitro antibacterial activity of Argentine folk medicinal plants against Salmonella typhi. Journal of Ethnopharmacology, 44(1), 41–46.
Clinical and laboratory Standards Institute. (2007). Performance standards for antimicrobial susceptibility testing. Approved Standard M100-S17. Approved Standard M100-S17. Clinical and Laboratory Standards Institute, Wayne, PA.
Clinical and laboratory Standards Institute (2008). Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A3 (3rd ed., ). Wayne, PA: Clinical and Laboratory Standards Institute.
Mishra ,. B., Leishangthem ,. G. D., Gill ,. K., Singh ,. A. K., Das ,. S., Singh ,. K., Xess ,. I., Dinda ,. A., Kapil ,. A., Patro ,. I. K., & Dey ,. S. (2013). A novel antimicrobial peptide derived from modified N-terminal domain of bovine lactoferrin: design, synthesis, activity against multidrug-resistant bacteria and Candida. Biochimica et Biophysica Acta, 1828(2), 677–686.
Kondori N., Baltzer L., Dolphin G. T., & Mattsby-Baltzer I. (2011). Fungicidal activity of human lactoferrin-derived peptides based on the antimicrobial αβ region. International Journal of Antimicrobial Agents, 37, 51–57.
Kumar S., Kapoor V., Gill K., Singh K., Xess I., Das S. N., & Dey S. (2014). Antifungal and antiproliferative protein from Cicer arietinum: a bioactive compound against emerging pathogens. BioMed Research International, 2014, 387203. doi:10.1155/2014/387203.
Dutta I., Saha P., Majumder P., Sarkar A., Chakraborti D., Banerjee S., & Das S. (2005). The efficacy of a novel insecticidal protein, Allium sativum leaf lectin (ASAL), against homopteran insects monitored in transgenic tobacco. Plant Biotechnology Journal, 3, 601–611.
Damme E. J. M. V., Smeets K., Engelborghs I., Aelbers H., Balzarini J., Pusztai A., Leuven F. V., Goldstein I. J., & Peumans W. J. (1993). Cloning and characterization of the lectin cDNA clones from onion, shallot and leek. Plant Molecular Biology, 23, 365–376.
Damme E. J. M. V., Smeets K., Torrekens S., Leuven F. V., Goldstein I. J., & Peumans W. J. (1992). The closely related homomeric and heterodimeric mannose-binding lectins from garlic are encoded by one-domain and two-domain lectin genes, respectively. European Journal of Biochemistry, 206, 413–420.
Smeets, K., Damme, E.J.M.V., Verhaert. P., Barre, A., Rouge, P., Leuven, F.V., &Peumans, W.J. (1997). Isolation, characterization and molecular cloning of the mannose-binding lectins from leaves and roots of garlic (Allium sativum L.). Plant Molecular Biology, 33, 223–234.
Zaika L. A., & Kissinger J. C. (1983). Inhibitory and stimulatory effects of oregano on Lactobacillus plantarum and Pediococcus cerevisiae. Journal of Food Science, 46, 1205–1210.
Whitemore B., & Naidu A. (2000). Natural food antimicrobial systems (pp. 265–380). Boca Raton, Florida, USA: CRC.
Cavallito C. J., & Bailey J. H. (1944). Allicin, the antibacterial principle of Allium sativum, isolation, physical properties and antibacterial action. Journal of the American Chemical Society, 66, 1950–1951.
Lund T., Stokke T., Olsen O. E., & Fodstad O. (2005). Garlic arrests MDA-MB-435 cancer cells in mitosis, phosphorylates the proapoptotic BH3-only protein BimEL and induces apoptosis. British Journal of Cancer, 92, 1773–1781.
Acknowledgments
The authors acknowledge the Indian Council of Medical Research, Government of India, New Delhi, India, for providing funds for the consumable items and Electron Microscopic Facility, AIIMS, for EM studies.
Ethics
The Ethics Committee of All India Institute of Medical Sciences, (AIIMS) New Delhi, India, approved the study protocol (IEC/NP-374/2013) and informed consent was obtained.
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 53 kb)
Rights and permissions
About this article
Cite this article
Kumar, S., Jitendra, K., Singh, K. et al. Biological Properties and Characterization of ASL50 Protein from Aged Allium sativum Bulbs. Appl Biochem Biotechnol 176, 1914–1927 (2015). https://doi.org/10.1007/s12010-015-1687-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1687-y