Abstract
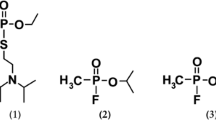
Paraoxonase 1 (PON1) is an important hydrolase, and the enzyme activity decreases in patients with liver disease, diabetes, coronary heart disease, etc. Phenyl acetate and organophosphates are usually employed as substrates for serum PON1 activity assay. However, phenyl acetate for arylesterase activity assay exhibits disadvantage of high background. According to properties of PON1, four new chemiluminescent acridinium esters were designed, prepared through three steps, and characterized with 1H NMR and mass spectrometry (MS) data, and their properties as PON1 substrates were investigated. The hydrolyses of the four compounds catalyzed by recombinant human PON1 (rhPON1) (or serum) followed first-order kinetics within 22 min. The PON1 activator (NaCl, 0.10 mol L−1) could boost the rhPON1-mediated and serum-mediated hydrolyses of the acridinium esters to 2.01 ~ 2.26 folds, but 1.0 mol L−1 NaCl decreased the serum arylesterase activity. RhPON1 showed selectivity over other serum esterases such as lipase, acetylcholinesterase, and esterase D more than 300 folds. By using ethylene diamine tetraacetic acid (EDTA) inhibitor, the specificities of the four substrates toward serum PON1 were determined as 78.3 ~ 92.9 %, which is improved than that of the model compound 9-(4-chloro-phenoxycarbonyl)-10-methylacridinium ester triflate. Due to low toxicity, high specificity, and sensitivity of the substrates, they are useful for serum PON1 activity assay.









Similar content being viewed by others
Abbreviations
- PON1:
-
Paraoxonase 1
- rhPON1:
-
Recombinant human PON1
- CL:
-
Chemiluminescent/chemiluminescence
- CPOCMA:
-
9-(4-chlorophenoxycarbonyl)-10-methylacridinium triflate ester
- EDTA:
-
Ethylene diamine tetraacetic acid
References
Durrington, P. N., Mackness, B., & Mackness, M. I. (2001). Paraoxonase and atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology, 21, 473–480.
Gan, K., Smolen, A., Eckerson, H. W., & La Du, B. N. (1991). Purification of human serum paraoxonase arylesterase-evidence for one esterase catalyzing both activities. Drug Metabolism and Disposition, 19, 100–106.
Mackness, M. I., Arrol, S., & Durrington, P. N. (1991). Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Letters, 286, 152–154.
Aydin, S., Sahin, I., Aydin, S., Aksoy, A., & Citil, C. (2012). The past and present of paraoxonase enzyme: its role in the cardiovascular system and some diseases. Journal of Medical Biochemistry, 31, 161–173.
Mehdi, M. M., & Rizvi, S. I. (2012). Human plasma paraoxonase 1 (PON1) arylesterase activity during aging: correlation with susceptibility of LDL oxidation. Archives of Medical Research, 43, 438–443.
Caraballo, J. C., Borcherding, J., Rector, M., Hornick, E., Stoltz, D., Zabner, J., & Comellas, A. P. (2014). Role of PON in anoxia-reoxygenation injury: a drosophila melanogaster transgenic model. PLoS One, 9, e84434.
Bonello, L., Bonello, N., Grosdidier, C., & Camoin-Jau, L. (2011). Unveiling the mysteries of clopidogrel metabolism and efficacy. Clinical Pharmacology and Therapeutics, 90, 774–776.
Hioki, T., Fukami, T., Nakajima, M., & Yokoi, T. (2011). Human paraoxonase 1 is the enzyme responsible for pilocarpine hydrolysis. Drug Metabolism and Disposition, 39, 1345–1352.
Saade, M., Magdalou, J., Ouaini, N., & Greige-Gerges, H. (2009). Stability of cucurbitacin E in human plasma: chemical hydrolysis and role of plasma esterases. Biopharmaceutics and Drug Disposition, 30, 389–397.
Macharia, M., Hassan, M. S., Blackhurst, D., Erasmus, R. T., & Matsha, T. E. (2012). The growing importance of PON1 in cardiovascular health: a review. Journal of Cardiovascular Medicine, 13, 443–453.
Goswami, B., Tayal, D., Gupta, N., & Mallikaet, V. (2009). Paraoxonase: a multifaceted biomolecule. Clinica Chimica Acta, 410, 1–12.
Richter, R. J., Jarvik, G. P., & Furlong, C. E. (2009). Paraoxonase 1 (PON1) status and substrate hydrolysis. Toxicology and Applied Pharmacology, 235, 1–9.
Pera-Kajan, J., & Jakubowski, H. (2012). Paraoxonase 1 and homocysteine metabolism. Amino Acids, 43, 1405–1417.
Costa, L. G., Giordano, G., & Furlong, C. E. (2011). Pharmacological and dietary modulators of paraoxonase 1 (PON1) activity and expression: the hunt goes on. Biochemical Pharmacology, 81, 337–344.
Ceron, J. J., Tecles, F., & Tvarijonaviciute, A. (2014). Serum paraoxonase 1 (PON1) measurement: an update. BMC Veterinary Research, 10, e74.
Camps, J., Marsillach, J., & Joven, J. (2009). The paraoxonases: role in human diseases and methodological difficulties in measurement. Critical Reviews in Clinical Laboratory Sciences, 46, 83–106.
Costa, L. G., Cole, T. B., Vitalone, A., & Furlong, C. E. (2005). Measurement of paraoxonase (PON1) status as a potential biomarker of susceptibility to organophosphate toxicity. Clinica Chimica Acta, 352, 37–47.
Soukharev, S., & Hammond, D. J. (2004). A fluorogenic substrate for detection of organophosphatase activity. Analytical Biochemistry, 327, 140–148.
Mu, X., Yu, N., Wang, C., Zou, X., Abulimite, Z., & Xia, Z. (2012). Evaluation of a new substrate for measurement of serum PON arylesterase activity. Talanta, 88, 711–716.
Mu, X. (2006). Synthesis of chemiluminescent compounds. PhD thesis. Swansea: Swansea University.
Ben-David, M., Elias, M., Filippi, J., Duñach, E., Silman, I., Sussman, J. L., & Tawfik, D. S. (2012). Catalytic versatility and backups in enzyme active sites: the case of serum paraoxonase 1. Journal of Molecular Biology, 418, 181–196.
Eckerson, H. W., Wyte, C. M., & La Du, B. N. (1983). The human serum paraoxonase/arylesterase polymorphism. American Journal of Human Genetics, 35, 1126–1138.
Haagen, L., & Brock, A. (1992). A new automated-method for phenotyping arylesterase (EC 3112) based upon inhibition of enzymatic-hydrolysis of 4-nitrophenyl acetate by phenyl acetate. European Journal of Clinical Chemistry and Clinical Biochemistry, 30, 391–395.
Krzyminski, K., Ozog, A., Malecha, P., Roshal, A. D., Wroblewska, A., Zadykowicz, B., & Blazejowski, J. (2011). Chemiluminogenic features of 10-methyl-9-(phenoxycarbonyl)acridinium trifluoromethanesulfonates alkyl substituted at the benzene ring in aqueous media. Journal of Organic Chemistry, 76, 1072–1085.
Primo-Parmo, S. L., Sorenson, R. C., Teiber, J., & La Du, B. N. (1996). The human serum paraoxonase arylesterase gene (PON1) is one member of a multigene family. Genomics, 33, 498–507.
Draganov, D. I., Teiber, J. F., Speelman, A., Osawa, Y., Sunahara, R., & La Du, B. N. (2005). Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. Journal of Lipid Research, 46, 1239–1247.
Ng, C. J., Wadleigh, D. J., Gangopadhyay, A., Hama, S., Grijalva, V. R., Navab, M., Fogelman, A. M., & Reddy, S. T. (2001). Paraoxonase-2 is a ubiquitously expressed protein with antioxidant properties and is capable of preventing cell-mediated oxidative modification of low density lipoprotein. Journal of Biological Chemistry, 276, 44444–44449.
Aviram, M., & Rosenblat, M. (2004). Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radical Biology and Medicine, 37, 1304–1316.
Draganov, D. I., Stetson, P. L., Watson, C. E., Billecke, S. S., & La Du, B. N. (2000). Rabbit serum paraoxonase 3 (PON3) is a high density lipoprotein-associated lactonase and protects low density lipoprotein against oxidation. Journal of Biological Chemistry, 275, 33435–33442.
La Du, B. N. (2001). Is paraoxonase-3 another HDL-associated protein protective against atherosclerosis? Arteriosclerosis, Thrombosis, and Vascular Biology, 21, 467–468.
Fukami, T., & Yokoi, T. (2012). The emerging role of human esterases. Drug Metabolism and Pharmacokinetics, 27, 466–477.
Bahar, F. G., Ohura, K., Ogihara, T., & Imai, T. (2012). Species difference of esterase expression and hydrolase activity in plasma. Journal of Pharmaceutical Sciences, 101, 3979–3988.
Parra, S., Alonso-Villaverde, C., Coll, B., Ferre, N., Marsillach, J., Aragones, G., Mackness, M., Mackness, B., Masana, L., Joven, J., & Camps, J. (2007). Serum paraoxonase-1 activity and concentration are influenced by human immunodeficiency virus infection. Atherosclerosis, 194, 175–181.
Grenner, G., Deutsch, G., Schmidtberger, R., & Dati, F. (1982). A highly sensitive enzyme-immunoassay for the determination of pancreatic lipase. Journal of Clinical Chemistry and Clinical Biochemistry, 20, 515–519.
Lee, W., Wheatley, W., Benedict, W. F., Huang, C., & Lee, E. (1986). Purification, biochemical-characterization, and biological function of human esterase-D. Proceedings of the National Academy of Sciences of the United States of America, 83, 6790–6794.
López-Flores, I., Lacasãna, M., Blanco-Muñoz, J., Aguilar-Gardữno, C., Sanchez-Villegasb, P., Pérez-Méndez, O. A., & Gamboa-avilad, R. (2009). Relationship between human paraoxonase-1 activity and PON1 polymorphisms in Mexican workers exposed to organophosphate pesticides. Toxicological Letters, 188, 84–90.
Ahmad, S., Carter, J. J., & Scott, J. E. (2010). A homogeneous cell-based assay for measurement of endogenous paraoxonase 1 activity. Analytical Biochemistry, 400, 1–9.
Pla, A., Rodrigo, L., Hernández, L., Gil, F., & Lopez, O. (2007). Effect of metal ions and calcium on purified PON1 and PON3 from rat liver. Chemico-Biological Interactions, 167, 63–70.
La Du, B. N. (1988). The human serum paraoxonase/arylesterase polymorphism. American Journal of Human Genetics, 43, 227–229.
Acknowledgments
This work was financially funded by Natural Science Foundation of Chongqing China (Nos. cstc2014jcsfglyjs0013, cstc2011BB5090) and NSFC (No.20805060). Also, thanks are given to the hospital of Chongqing University for kindly collecting human blood serum samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abulimite, Z., Mu, X., Xiao, S. et al. New Chemiluminescent Substrates of Paraoxonase 1 with Improved Specificity: Synthesis and Properties. Appl Biochem Biotechnol 176, 301–316 (2015). https://doi.org/10.1007/s12010-015-1575-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1575-5