Abstract
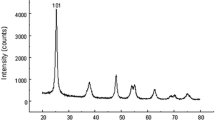
Serious concerns have been expressed about potential risks of engineered nanoparticles. Regulatory health risk assessment of such particles has become mandatory for the safe use in consumer products and medicines; also, the potential effects on reproduction and fertility are relevant for this risk evaluation. In the present study, we examined the effects of intravenously injected titanium dioxide nanoparticles (TiO2-NPs; 21 nm), with special emphasis on reproductive system. Antioxidant enzymes such as catalase, glutathione peroxidase, and superoxide dismutase showed a significant decrease, while significant increase in lipid peroxidase was observed. Our results confirmed the bioaccumulation of TiO2-NPs in testicular cells. In TiO2-NPs-treated animals, various functional and pathological disorders, such as reduced sperm count, increase in caspase-3 (a biomarker of apoptosis), creatine kinase activity, DNA damage, and cell apoptosis were observed. Moreover, the testosterone activity was decreased significantly in a dose-dependent manner in the animals treated with TiO2-NPs as compared with control group animals. It is concluded that TiO2-NPs induce oxidative stress, which produce cytotoxic and genotoxic changes in sperms which may affect the fertilizing potential of spermatozoa.













Similar content being viewed by others
References
Mazzola, L. (2003). Commercialising nanotechnology. Nature Biotechnology, 21, 1137–1143.
Paull, R., Wolfe, J., Hebert, P., & Sinkula, M. (2003). Investing in nanotechnology. Nature Biotechnology, 21, 1144–1147.
Salata, O. V. (2004). Applications of nanoparticles in biology and medicine. Journal Nanobiotechnology, 2, 3.
Nel, A., Xia, T., Madler, L., & Li, N. (2006). Toxic potential of nanomaterials at the nanolevel. Science, 311, 622–627.
Donaldson, K., Stone, V., Tran, C. L., Kreyling, W., & Borm, P. J. A. (2004). Nanotoxicology. Occupational and Environmental Medicine, 61, 727–728.
Hoet, P. H. M., Bruske-Hohlfeld, I., & Salata, O. V. (2004). Nanoparticles known and unknown health risks. Journal Nanobiotechnology, 2, 12.
Oberdorster, G., Oberdorster, E., & Oberdorster, J. (2005). Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environmental Health Perspectives, 113, 823–839.
Newman, M. D., Stotland, M., & Ellis, J. I. (2009). The safety of nanosized particles in titanium dioxide–and zinc oxide–based sunscreens. Journal of the American Academy of Dermatology, 61(4), 685–692.
Meena, R., & Paulraj, R. (2012). Oxidative stress mediated cytotoxicity of TiO2 nano anatase in liver and kidney of Wistar rat. Toxicological and Environmental Chemistry, 94(1), 146–163.
Gurr, J., Wang, A. A. S., Chen, C., & Jan, K. (2005). Ultrafine titanium dioxide particles in the absence of photoactivation can induce oxidative damage to human bronchial epithelial cells. Toxicology, 213, 66–73.
De Jong, W. H., Hagens, W. I., Krystek, P., Burger, M. C., Sips, A. J., & Geertsma, R. E. (2008). Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials, 29, 1912–1919.
Lankveld, D. P., Oomen, A. G., Krystek, P., Neigh, A., Troost-de, J. A., Noorlander, C. W., Van Eijkeren, J. C., Geertsma, R. E., & De Jong, W. H. (2010). The kinetics of the tissue distribution of silver nanoparticles of different sizes. Biomaterials, 31, 8350–8361.
Yoshida, S., Ono, N., Tsukue, N., Oshio, S., Umeda, T., Takano, H., & Takeda, K. (2006). In utero exposure to diesel exhaust increased accessory reproductive gland weight and serum testosterone concentration in male mice. Environmental Sciences, 13, 139–147.
Asare, N., Instanesa, C., Sandberga, W. J., Refsnesa, M., Schwarzea, P., Kruszewskib, M., & Brunborg, G. (2012). Cytotoxic and genotoxic effects of silver nanoparticles in testicular cells. Toxicology, 291, 65–72.
Braydich-Stolle, L., Hussain, S., Schlager, J. J., & Hofmann, M. C. (2005). In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicological Sciences, 88(2), 412–419.
Ema, M., Kobayashi, N., Naya, M., Hanai, S., & Nakanishi, J. (2010). Reproductive and developmental toxicity studies of manufactured nanomaterials. Reproductive Toxicology, 30, 343–352.
Chen, Y., Xue, Z., Zheng, D., Xia, K., Zhao, Y., Liu, T., Long, Z., & Zia, J. (2003). Sodium chloride modified silica nanoparticles as a non viral vector with a high efficiency of DNA transfer into cells. Current Gene Therapy, 3, 273–279.
Kim, J. S., Sung, J. H., Ji, J. H., Song, K. S., Lee, J. H., Kang, C. S., & Yu, I. J. (2011). In vivo genotoxicity of silver nanoparticles after 90-day silver nanoparticle inhalation exposure. Safety Health Work, 2, 34–38.
Cayli, S., Sakkas, D., Vigue, L., Demir, R., & Huszar, G. (2004). Cellular maturity and apoptosis in human sperm: creatine kinase, caspase-3 and Bcl-XL levels in mature and diminished maturity sperm. Molecular Human Reproduction, 10, 365–372.
Ceruti, S., Beltrami, E., Matarrese, P., Mazzola, A., Cattabeni, F., & Malorni, W. (2003). A key role for caspase-2 and caspase-3 in the apoptosis induced by 2-chloro-2'-deoxy-adenosine (cladribine) and 2-chloro-adenosine in human astrocytoma cells. Molecular Pharmacology, 63, 1437–1447.
Riedl, S. J., & Shi, Y. (2004). Molecular mechanisms of caspase regulation during apoptosis. Nature Reviews Molecular Cell Biology, 5, 897–907.
Pommier, Y., Sordet, O., Antony, S., Hayward, R. L., & Kohn, K. W. (2004). Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene, 23, 2934–2949.
Hallak, J., Sharma, R. K., Pasqualotto, F. F., Ranganathan, P., Thomas, A. J., & Agarwal, A. (2001). Creatine kinase as an indicator of sperm quality and maturity in men with oligospermia. Urology, 58, 446–451.
Wallimann, T., Moser, H., Zurbriggen, B., Wegmann, G., & Eppenberger, H. M. (1986). Creatine kinase isoenzymes in spermatozoa. Journal of Muscle Research and Cell Motility, 7, 25–34.
Kavanagh, J. P., & Darby, C. (1983). Creatine kinase and ATPase in human seminal fluid and prostatic fluid. Journal of Reproduction and Fertility, 68, 51–56.
Huszar, G., Corrales, M., & Vigue, L. (1988). Correlation between sperm creatine phosphokinase activity and sperm concentrations in normospermic and oligospermic men. Gamete Research, 19, 67–75.
Huszar, G., Vigue, L., & Corrales, M. (1988). Sperm creatine phosphokinase activity as a measure of sperm quality in normospermic, variablespermic, and oligospermic men. Biology of Reproduction, 38, 1061–1066.
Huszar, G., & Vigue, L. (1993). Incomplete development of human spermatozoa is associated with increased creatine phosphokinase concentration and abnormal head morphology. Molecular Reproduction and Development, 34, 292–298.
Marklund, S., & Marklund, G. (1974). Involvement of the superoxide anion radical in autoxidation of pyrogallol as a convenient assay for superoxide dismutase. European Journal of Biochemistry, 47, 469–474.
Aebi, H. (1974). Catalase. In Bergmeyer (ed.), Methods of Enzymatic. Analysis, 2, 673–684. New York: Academic Press.
Mills, G. C. (1959). The purification and properties of glutathione peroxidase of erythrocytes. Journal of Biological Chemistry, 234, 502.
Varshney, R., & Kale, R. K. (1990). Effect of calmodulin antagonist on radiation-induced lipid peroxidation in microsomes. International Journal of Radiation Biology, 58, 733–743.
Kumar, S., Kesari, K. K., & Behari, J. (2011). The therapeutic effect of a pulsed electromagnetic field on the reproductive patterns of male Wistar rats exposed to a 2.45-GHz microwave field. Clinics, 66(7), 1237–1245.
Paulraj, R., & Behari, J. (2006). Single strand DNA breaks in rat brain cells exposed to microwave radiation. Mutation Research, 596, 76–80.
Gong, J. P., Traganos, F., & Darzynkiewicz, Z. (1994). A selective procedure for DNA extraction from apoptotic cells applicable for gel electrophoresis and flow cytometry. Analytical Biochemistry, 218(2), 314–319.
Tiwari, D. K., Jin, T., & Behari, J. (2011). Dose-dependent in-vivo toxicity assessment of silver nanoparticle in Wistar rats. Toxicology Mechanisms and Methods, 21(1), 13–24.
Simeonova, P. P., & Lusster, M. T. (1995). Iron and reactive oxygen species in the asbestos-induced tumour necrosis factor a response from alveolar macrophages. American Journal of Respiratory Cell and Molecular Biology, 12(6), 676–683.
Zhao, X., Sheng, L., Wang, L., Hong, J., Yu, X., Sang, X., & Hong, F. (2014). Mechanisms of nanosized titanium dioxide-induced testicular oxidative stress and apoptosis in male mice. Particle and Fibre Toxicology, 11(1), 47.
Chaudhari, M., Jayaraj, R., Bhaskar, A. S. B., & Lakshmana, R. (2009). Oxidative stress induction by T-2 toxin causes DNA damage and triggers apoptosis via caspase pathway in human cervical cancer cells. Toxicology, 262, 153–161.
Da Silva Paula, M. M., da Costa, C. S., Baldin, M. C., Scaini, G., Rezin, G. T., Segala, K., & Streckb, E. L. (2009). In vitro effect of silver nanoparticles on creatine kinase activity. Journal of the Brazilian Chemical Society, 20(8), 1556–1560.
Hemmati, P. G., Normand, G., Gillissen, B., Wendt, J., Dorken, B., & Daniel, P. T. (2008). Cooperative effect of p21Cip1/WAF-1 and 14-3-3sigma on cell cycle arrest and apoptosis induction by p14ARF. Oncogene, 27, 6707–6719.
Guo, L. L., Liu, X. H., Qin, D. X., Gao, L., Zhang, H. M., Liu, J. Y., & Cui, Y. G. (2009). Effects of nanosized titanium dioxide on the reproductive system of male mice. National Journal of Andrology, 15(6), 517.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meena, R., Kajal, K. & R., P. Cytotoxic and Genotoxic Effects of Titanium Dioxide Nanoparticles in Testicular Cells of Male Wistar Rat. Appl Biochem Biotechnol 175, 825–840 (2015). https://doi.org/10.1007/s12010-014-1299-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1299-y