Abstract
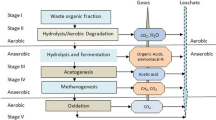
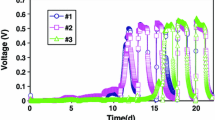
Microbial fuel cells were designed and operated to treat landfill leachate while simultaneously producing electricity. Two designs were tested in batch cycles using landfill leachate as a substrate without inoculation (908 to 3,200 mg/L chemical oxygen demand (COD)): Circle (934 mL) and large-scale microbial fuel cells (MFC) (18.3 L). A total of seven cycles were completed for the Circle MFC and two cycles for the larger-scale MFC. Maximum power densities of 24 to 31 mW/m2 (653 to 824 mW/m3) were achieved using the Circle MFC, and a maximum voltage of 635 mV was produced using the larger-scale MFC. In the Circle MFC, COD, biological oxygen demand (BOD), total organic carbon (TOC), and ammonia were removed at an average of 16%, 62%, 23%, and 20%, respectively. The larger-scale MFC achieved an average of 74% BOD removal, 27% TOC removal, and 25% ammonia reduction while operating over 52 days. Analysis of the microbial characteristics of the leachate indicates that there might be both supportive and inhibiting bacteria in landfill leachate for operation of an MFC. Issues related to scale-up and heterogeneity of a mixed substrate remain.



Similar content being viewed by others
References
Aelterman, P., Versichele, M., Marzorati, M., Boon, N., & Verstraete, W. (2008). Loading rate and external resistance control the electricity generation of microbial fuel cells with different three-dimensional anodes. Bioresource Technology, 99, 8895–8902.
American Public Health Association (APHA), American Water Works Association, and Water Environment Federation, (1998). Standard Methods for the Examination of Water and Wastewater, American Public Health Association, American Water Works Association, 20th Edition.
Barlaz, M. A., Rooker, A. P., Kjeldsen, P., Gabr, M. A., & Borden, R. C. (2002). Critical evaluation of factors required to terminate the postclosure monitoring period at solid waste landfills. Environmental Science and Technology, 36(16), 3457–3464.
Cheng, S., Liu, H., & Logan, B. E. (2006). Power densities using different cathode catalysts (Pt and CoTMPP) and polymer binders (Nafion and PTFE) in single chamber microbial fuel cells. Environmental Science and Technology, 40, 364–369.
Damiano, L. (2009). Electricity production from the management of municipal solid waste leachate with microbial fuel cells. In Masters thesis. Durham, NH: University of New Hampshire.
Damiano, L., & Jambeck, J. (2009). Leachate treatment and electricity production in microbial fuel cells. Sardinia, Italy: Proceedings of the Sardinia Waste Symposium. 2009.
Ganesh, K., & Jambeck, J. R. (2013). Treatment of landfill leachate using microbial fuel cells: Alternative anodes and semi-continuous operation. Bioresource Technology, 139, 383–387.
Feng, Y., Wang, X., Logan, B. E., & Lee, H. (2008). Brewery wastewater treatment using air-cathode microbial fuel cells. Environmental Biotechnology, 78, 873–880.
Gorby, Y. A., Yanina, S., McLean, J. S., Rosso, K. M., Moyles, D., Dohnaikova, A., Beveridge, T. J., Chang, I. D., Kim, B. H., Kim, K. S., Culley, D. E., Reed, S. B., Romine, M. F., Saffarinl, D. A., Hill, E. A., Shi, L., Ellas, D. A., Kennedy, D., Pinchuk, G., Watanabe, K., Ishll, S., Lodan, B., Nealson, K. H., & Fredrickson, J. K. (2006). Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proceedings of the National Academy of Sciences of the United States of America, 103(30), 11358–11363.
Greenman, J., Galvez, A., Giusti, L., & Ieropoulos, I. (2009). Electricity from landfill leachate using microbial fuel cells: Comparison with a biological aerated filter. Enzyme and Microbial Technology, 44, 112–119.
Hach, 2013a. Oxygen demand, chemical, dichromate method 8000 (multi-range: 40.0, 150, 1500, 15,000 mg/L), Hach Water Analysis Handbook, http://www.hach.com/asset-get.download.jsa?id=7639983816.
Hach, 2013b. Sulfide, methylene blue method 8131, Hach Water Analysis Handbook, http://www.hach.com/asset-get.download.jsa?id=7639983902.
Huang, L., & Logan, B. E. (2008). Electricity generation and treatment of paper recycling wastewater using a microbial fuel cell. Environmental Biotechnology, 80, 349–355.
Jambeck, J. R., Townsend, T. G., & Solo-Gabriele, H. M. (2008). Landfill disposal of CCA-treated wood with construction and demolition (C&D) debris: Arsenic, chromium, and copper concentrations in leachate. Environmental Science and Technology, 42(15), 5740–5745.
Kim, J. R., Cheng, S., Oh, S. E., & Logan, B. E. (2007). Power generation using different cation, anion, and ultrafiltration membranes in microbial fuel cells. Environmental Science and Technology, 41, 1004–1009.
Kim, J. R., Dec, J., Bruns, M. A., & Logan, B. E. (2008). Removal of odors from swine wastewater by using microbial fuel cells. Applied and Environmental Microbiology, 74(8), 2540–2543.
Kjeldsen, P., Barlaz, M. A., Rooker, A. P., Baun, A., Ledin, A., & Christensen, T. H. (2002). Present and long-term composition of MSW landfill leachate: A review. Critical Reviews in Environmental Science and Technology, 32(4), 297–336.
Li, Y., Lu, A., Ding, H., Wang, X., Wang, C., Zeng, C., & Yan, Y. (2010). Microbial fuel cells using natural pyrrhotite as the cathodic heterogeneous Fenton catalyst towards the degradation of biorefractory organics in landfill leachate. Electrochemistry Communications, 12(7), 944–947.
Liu, H., Ramarayanan, R., & Logan, B. E. (2004). Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environmental Science and Technology, 38, 2281–2285.
Liu, H., Cheng, S., & Logan, B. E. (2005). Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environmental Science and Technology, 39, 5488–5493.
Liu, H., Cheng, S., Huang, L., & Logan, B. E. (2008). Scale-up of membrane-free single chamber microbial fuel cells. Journal Power Sources, 179, 274–279.
Logan, B. E., & Regan, J. M. (2006). Electricity-producing bacterial communities in microbial fuel cells. TRENDS Mirobiology, 14(12), 512–518.
Logan, B. E., Hamelers, B., Rozendal, R., Schroder, U., Keller, J., Reguia, S., Aelterman, P., Verstraete, W., & Rabaey, K. (2006). Microbial fuel cells: Methodology and technology. Environmental Science and Technology, 40(17), 5181–5192.
Logan, B. E., Cheng, S., Watson, V., & Estadt, G. (2007). Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environmental Science and Technology, 41, 3341–3346.
Logan, B. E. (2008). Microbial fuel cells. New Jersey: John Wiley and Sons, Inc.
Lovley, D. R. (2006). Bug juice: Harvesting electricity with microorganisms. Nature Reviews, 4, 497–508.
Lee, Y., Martin, L., Grasel, P., Tawfiq, K., (2013). Power generation and nitrogen removal of landfill leachate using microbial fuel cell technology, Environ. Technol., doi.org/10.1080/09593330.2013.788040.
Min, B., & Logan, B. E. (2004). Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environmental Science and Technology, 38, 5809–5814.
Min, B., Kim, J. R., Oh, S. E., Regan, J. M., & Logan, B. E. (2005). Electricity generation from swine wastewater using microbial fuel cells. Water Research, 39, 4961–4968.
Oh, S. E., & Logan, B. E. (2006). Proton exchange membrane and electrode surface areas as factors that affect power generation in microbial fuel cells. Biotechnology Production Process Engineering, 70, 162–169.
Pfaff, J. D. (1993). METHOD 300.0 Determination of inorganic anions by ion chromatography, environmental monitoring systems laboratory. U.S. EPA: Office of Research and Development. http://water.epa.gov/scitech/methods/cwa/bioindicators/upload/2007_07_10_methods_method_300_0.pdf.
Puig, S., Serra, M., Coma, M., Cabré, M., Balaguer, M. D., & Colprim, J. (2011). Microbial fuel cell application in landfill leachate treatment. Journal of Hazardous Materials, 185, 763–767.
USEPA (United States Environmental Protection Agency) (2011a). Municipal solid waste generation, recycling, and disposal in the United States: Facts and figures for 2010; Washington, DC.
USEPA (United States Environmental Protection Agency), (2011b). Criteria for municipal solid waste landfills; Code of Federal regulations, Title 40, parts 257 and 258.
USEPA (United States Environmental Protection Agency), (2004). METHOD 9060A Total organic carbon, test methods for evaluating solid waste, physical/chemical methods (SW-846), http://www.epa.gov/waste/hazard/testmethods/sw846/online/.
USEPA, 1983. Methods for chemical analysis of water and wastes, EPA-600/4-79-020, revised 3/83, method 365.3.
Virdis, B., Rabaey, K., Rozendal, R. A., Yuan, Z., & Keller, J. (2010). Simultaneous nitrification, denitrification and carbon removal in microbial fuel cells. Water Research, 44, 2970–2980.
You, S. J., Zhao, Q. L., Jiang, J. Q., Zhang, J. N., & Zhao, S. Q. (2006). Sustainable approach for leachate treatment: Electricity generation in microbial fuel cell. Journal Environmental Science and Health Part A, 41, 2721–2734.
Acknowledgment
The authors acknowledge the donation of Waste Management’s Turnkey Landfill leachate for this work and the Environmental Research and Education Foundation for funding this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Damiano, L., Jambeck, J.R. & Ringelberg, D.B. Municipal Solid Waste Landfill Leachate Treatment and Electricity Production Using Microbial Fuel Cells. Appl Biochem Biotechnol 173, 472–485 (2014). https://doi.org/10.1007/s12010-014-0854-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-0854-x