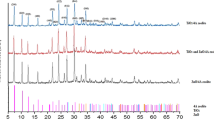
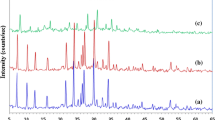
Abstract
Zeolites are nanoporous alumina silicates composed of silicon, aluminum, and oxygen in a framework with cations, water within pores. Their cation contents can be exchanged with monovalent or divalent ions. In the present study, the antimicrobial (antibacterial, anticandidal, and antifungal) properties of zeolite type X and A, with different Al/Si ratio, ion exchanged with Ag+, Zn2+, and Cu2+ ions were investigated individually. The study presents the synthesis and manufacture of four different zeolite types characterized by scanning electron microscopy and X-ray diffraction. The ion loading capacity of the zeolites was examined and compared with the antimicrobial characteristics against a broad range of microorganisms including bacteria, yeast, and mold. It was observed that Ag+ ion-loaded zeolites exhibited more antibacterial activity with respect to other metal ion-embedded zeolite samples. The results clearly support that various synthetic zeolites can be ion exchanged with Ag+, Zn2+, and Cu2+ ions to acquire antimicrobial properties or ion-releasing characteristics to provide prolonged or stronger activity. The current study suggested that zeolite formulations could be combined with various materials used in manufacturing medical devices, surfaces, textiles, or household items where antimicrobial properties are required.


Similar content being viewed by others
References
Chon, H., Woo, S. I., and Park, S. E. (1996), Elsevier, Amsterdam
Rožić, M., Cerjan-Stefanović, Š., Kurajica, S., Vančina, V., & Hodžić, E. (2000). Water Research, 34, 3675–3681.
Saint–Cricq, P., Kamimura, Y., Itabashi, K., Sugawara–Narutaki, A., Shimojima, A., and Okubo, T. (2012) European Journal of Inorganic Chemistry, 2012, 3398–3402
Win, D. T. (2012). AU Journal, 11, 36–41.
Inoue, Y., Hoshino, M., Takahashi, H., Noguchi, T., Murata, T., Kanzaki, Y., Hamashima, H., & Sasatsu, M. (2002). Journal of Inorganic Biochemistry, 92, 37–42.
Kralj, M., & Pavelic, K. (2003). EMBO Reports, 4, 1008–1012.
Abe, Y., Ishii, M., Takeuchi, M., Ueshige, M., Tanaka, S., & Akagawa, Y. (2004). Journal of Oral Rehabilitation, 31, 568–573.
Zhang, Y., Zhong, S., Zhang, M., & Lin, Y. (2009). Journal of Materials Science, 44, 457–462.
Kwakye-Awuah, B., Williams, C., Kenward, M., & Radecka, I. (2008). Journal of Applied Microbiology, 104, 1516–1524.
Ferreira, L., Fonseca, A. M., Botelho, G., Aguiar, C. A., & Neves, I. C. (2012). Microporous and Mesoporous Materials, 160, 126–132.
Hrenovic, J., Milenkovic, J., Ivankovic, T., & Rajic, N. (2012). Journal of Hazardous Materials, 201, 260–264.
Malachová, K., Praus, P., Rybková, Z., & Kozák, O. (2011). Applied Clay Science, 53, 642–645.
Niira, R., Yamamoto, T., and Uchida, M., (1990), Google patents
Top, A., & Ülkü, S. (2004). Applied Clay Science, 27, 13–19.
Hagiwara, Z., Hoshino, S., Ishino, H., Nohara, S., Tagawa, K., and Yamanaka, K., (1988), Google patents.
Kawahara, K., Tsuruda, K., Morishita, M., & Uchida, M. (2000). Dental Materials, 16, 452–455.
Sallam, M., (2006), University of South Florida
Casemiro, L. A., Martins, C. H. G., Pires-de-Souza, F. D. C., & Panzeri, H. (2008). Gerodontology, 25, 187–194.
Clement, J. L., & Jarrett, P. S. (1994). Metal Based Drugs, 1, 467–482.
Kamışoğlu, K., Aksoy, E., Akata, B., Hasirci, N., & Baç, N. (2008). Journal of Applied Polymer Science, 110, 2854–2861.
Mehtar, S., Wiid, I., & Todorov, S. (2008). Journal of Hospital Infection, 68, 45–51.
Rivera-Garza, M., Olguın, M., Garcıa-Sosa, I., Alcántara, D., & Rodrıguez-Fuentes, G. (2000). Microporous and Mesoporous Materials, 39, 431–444.
Kim, T., Feng, Q., Kim, J., Wu, J., Wang, H., Chen, G., & Cui, F. (1998). Journal of Materials Science. Materials in Medicine, 9, 129–134.
Baldrian, P., (2010) Effect of heavy metals on saprotrophic soil fungi. New York, NY, Springer, pp. 263–279
Acknowledgments
This research was supported by Yeditepe University. The authors deny any conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Demirci, S., Ustaoğlu, Z., Yılmazer, G.A. et al. Antimicrobial Properties of Zeolite-X and Zeolite-A Ion-Exchanged with Silver, Copper, and Zinc Against a Broad Range of Microorganisms. Appl Biochem Biotechnol 172, 1652–1662 (2014). https://doi.org/10.1007/s12010-013-0647-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0647-7