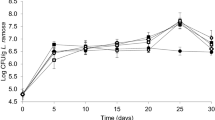
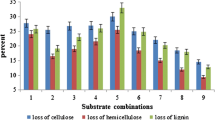
Abstract
Rapeseed meal is valuable high-protein forage, but its nutritional value is significantly reduced by the presence of a number of antinutrients, including phenolic compounds. Solid-state fermentation with white-rot fungi was used to decrease the sinapic acid concentration of rapeseed meal. After 7 days of growth of Trametes versicolor and Pleurotus ostreatus, the sinapic acid content of rapeseed meal was reduced by 59.9 and 74.5 %, respectively. At the end of the experiment, sinapic acid concentration of T. versicolor cultures decreased by 93 % of the initial value; in the case of cultures of P. ostreatus, 93.2 % reduction was observed. Moreover, cultivation of white-rot fungi on rapeseed meal resulted in the intensive production of extracellular laccase, particularly strong during the late phases of growth of T. versicolor. The obtained results confirm that both fungal species may effectively be used to decompose antinutritional phenolics of rapeseed meal. Rapeseed meal may also find use as an inexpensive and efficient substrate for a biotechnological production of laccase by white-rot fungi.



Similar content being viewed by others
References
Bell, J. M. (1984). Journal of Animal Science, 58, 996–1010.
Burel, C., Boujard, T., Tulli, F., & Kaushik, S. J. (2000). Aquaculture, 188, 285–298.
Vioque, J., Sánchez-Vioque, R., Clemente, A., Pedroche, J., & Millán, F. (2000). Journal of the American Oil Chemists' Society, 77, 447–450.
Vuorela, S., Meyer, A. S., & Heinonen, M. (2004). Journal of Agricultural and Food Chemistry, 52, 8202–8207.
Shahidi, F., & Naczk, M. (1992). Journal of the American Oil Chemists' Society, 69, 917–924.
Khattab, R., Eskin, M., Aliani, M., & Thiyam, U. (2010). Journal of the American Oil Chemists' Society, 87, 147–155.
Lacki, K., & Duvnjak, Z. (1999). Biotechnology and Bioengineering, 62, 422–433.
Kirk, T. K., & Cullen, D. (1998). In R. A. Young & M. Akhtar (Eds.), Environmentally friendly technologies for the pulp and paper industry (pp. 237–307). New York: Wiley.
Shah, V., & Nerud, F. (2002). Canadian Journal of Microbiology, 48, 857–870.
Isroi, Millati, R., Syamsiah, S., Niklasson, C., Cahyanto, M. N., Lundquist, K., & Taherzadeh, M. J. (2011). BioResources, 6, 5224–5259.
Hu, J., & Duvnjak, Z. (2004). Engineering in Life Sciences, 4, 50–55.
Smith, J. E., Rowan, N. J., & Sullivan, R. (2002). Biotechnology Letters, 24, 1839–1845.
Lindeberg, G., & Holm, G. (1952). Physiologia Plantarum, 5, 100–114.
Thiyam, U., Stöckmann, H., & Schwarz, K. (2006). Journal of the American Oil Chemists' Society, 83, 523–528.
Lundell, T., Leonowicz, A., Rogalski, J., & Hatakka, A. (1990). Applied and Environmental Microbiology, 56, 3515–3520.
Jaszek, M., Żuchowski, J., Dajczak, K., Cimek, M., Grąz, M., & Grzywnowicz, K. (2006). International Biodeterioration & Biodegradation, 58, 168–175.
Kwang-Soo, S., & Chang-Jin, K. (1998). Biotechnology Techniques, 12, 101–104.
Lacki, K., & Duvnjak, Z. (1998). Biotechnology and Bioengineering, 57, 694–703.
Koroleva, O. V., Gavrilova, V. P., Stepanova, E. V., Lebedeva, V. I., Sverdlova, N. I., Landesman, E. O., Yavmetdinov, I. S., & Yaropolov, A. I. (2002). Enzyme and Microbial Technology, 30, 573–580.
Bau, H.-M., Villaume, C., Lin, C.-F., Evrard, J., Quemener, B., Nicolas, J.-P., & Méjean, L. (1994). Journal of the Science of Food and Agriculture, 65, 315–322.
Vig, A. P., & Walia, A. (2001). Bioresources Technology, 78, 309–312.
Al-Asheh, S., & Duvnjak, Z. (1995). World Journal of Microbiology and Biotechnology, 11, 228–231.
El-Batal, A. I., & Abdel Karem, H. (2001). Food Research International, 34, 715–720.
Acknowledgments
The authors would like to thank Prof. Jerzy Rogalski for his kind consent for the use of fungal strains from the culture collection of the Department of Biochemistry, M. Curie-Skłodowska University in Lublin, Poland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Żuchowski, J., Pecio, Ł., Jaszek, M. et al. Solid-State Fermentation of Rapeseed Meal with the White-Rot Fungi Trametes versicolor and Pleurotus ostreatus . Appl Biochem Biotechnol 171, 2075–2081 (2013). https://doi.org/10.1007/s12010-013-0506-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0506-6