Abstract
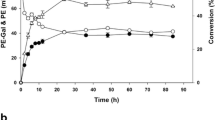
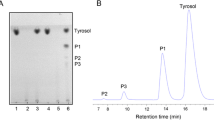
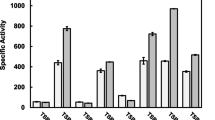
We synthesized galactosyl chlorphenesin (CPN-G) using β-gal-containing Escherichia coli (E. coli) cells in which the conversion yield of chlorphenesin (CPN) to CPN-G reached about 64 % during 12 h. CPN-G was identified and characterized using high-performance liquid chromatography, liquid chromatography-mass spectrometry, Fourier transform-infrared spectrometry, and nuclear magnetic resonance analysis (1H and 13C). We verified that a galactose was covalently bound to a CPN alcohol group during CPN-G synthesis throughout these analyses. In particular, by the hydrolysis of CPN-G using β-gal, it was confirmed that a galactose was bound to CPN. The minimal inhibitory concentration (MIC) results showed that the CPN-G MICs were fairly similar to those of CPN. HACAT cell viability was significantly higher in CPN-G-treated cells than in CPN-treated cells at concentrations of 0.0–20.0 mM. Finally, we accomplished the synthesis of less toxic CPN-G, compared with CPN, using β-gal-containing E. coli cells as whole cells without changes in the MICs against microorganisms.






Similar content being viewed by others
References
Harju, M., Kallioinen, H., & Tossavainen, O. (2012). Lactose hydrolysis and other conversions in dairy products: technological aspects. International Dairy Journal, 22, 104–109.
Gänzle, M. G. (2012). Enzymatic synthesis of galacto-oligosaccharides and other lactose derivatives (hetero-oligosaccharides) from lactose. International Dairy Journal, 22, 116–122.
Melisi, D., Curcio, A., Luongo, E., Morelli, E., & Rimoli, M. G. (2011). D-Galactose as a vector for prodrug design. Current Topics in Medicinal Chemistry, 11, 2288–2298.
Benjamin, G. D., & Robinson, M. A. (2002). Drug delivery systems based on sugar-macromolecule conjugates. Current Opinion in Drug Discovery & Development, 5, 279–288.
Huang, J., Gao, F., Tang, X., Yu, J., Wang, D., Liu, S., et al. (2010). Liver-targeting doxorubicin-conjugated polymeric prodrug with pH-triggered drug release profile. Polymer International, 59, 1390–1396.
Tietze, L. F., & Schmuck, K. (2011). Prodrugs for targeted tumor therapies: recent developments in ADEPT, GDEPT and PMT. Current Pharmaceutical Design, 17, 3527–3547.
Kim, G.-E., Lee, J.-H., Jung, S.-H., Seo, E.-S., Jin, S.-D., Kim, G. J., et al. (2010). Enzymatic synthesis and characterization of hydroquinone galactoside using Kluyveromyces lactis lactase. Journal of Agricultural and Food Chemistry, 58, 9492–9497.
Lu, L., Xu, X., Gu, G., Jin, L., Xiao, M., & Wang, F. (2010). Synthesis of novel galactose containing chemicals by β-galactosidase from Enterobacter cloacae B5. Bioresource Technology, 101, 6868–6872.
Moil, T., Fujita, S., & Okahata, Y. (1997). Transglycosylation in a two-phase aqueous-organic system with catalysis by a lipid-coated β-D-galactosidase. Carbohydrate Research, 298, 65–73.
Vera, C., Guerrero, C., & Illanes, A. (2011). Determination of the transgalactosylation activity of Aspergillus oryzae β-galactosidase: effect of pH, temperature, and galactose and glucose concentrations. Carbohydrate Research, 346, 745–752.
Kwon, S. K., Jung, H.-C., & Pan, J.-G. (2007). Transgalactosylation in a water-solvent biphasic reaction system with β-galactosidase displayed on the surfaces of Bacillus subtilis spores. Applied and Environmental Microbiology, 73, 2251–2256.
Badarinath, V., & Halami, P. M. (2011). Purification of new β-galactosidase from Enterococcus faecium MTCC 5153 with transgalactosylation activity. Food Biotechnology, 25, 225–239.
Liu, G. X., Kong, J., Lu, W. W., Kong, W. T., Tian, H., Tian, X. Y., et al. (2011). β-Galactosidase with transgalactosylation activity from Lactobacillus fermentum K4. Journal of Dairy Science, 94, 5811–5820.
Lee, S.-E., Seo, H.-B., Kim, H.-J., Yeon, J.-H., & Jung, K.-H. (2011). Galactooligosacchride synthesis by active β-galactosidase inclusion bodies-containing Escherichia coli cells. Journal of Microbiology and Biotechnology, 21, 1151–1158.
Jung, K. H. (2008). Enhanced enzyme activities of inclusion bodies of recombinant β-galactosidase via the addition of inducer analog after L-arabinose induction in the araBAD promoter system of Escherichia coli. Journal of Microbiology and Biotechnology, 18, 434–442.
Asraf, S. S., & Gunasekaran, P. (2010). Current trends of ß-galactosidase research and application. In A. Mendez-Vilas (Ed.), Current research, technology and education topics in applied microbiology and microbial biotechnology (pp. 880–890). Badajoz: Formatex Research Center.
Oliveira, C., Guimarães, P. M. R., & Domingues, L. (2011). Recombinant microbial systems for improved β-galactosidase production and biotechnological applications. Biotechnology Advances, 29, 600–609.
Dugdale, M. L., Dymianiw, D. L., Minhas, B. K., D’Angelo, I., & Huber, R. E. (2010). Role of Met-542 as a guide for the conformational changes of Phe-601 that occur during the reaction of β-galactosidase (Escherichia coli). Biochemistry and Cell Biology, 88, 861–869.
Lo, S., Dugdale, M. L., Jeerh, N., Ku, T., Roth, N. J., & Huber, R. E. (2010). Studies of Glu-416 variants of β-galactosidase (E. coli) show that the active site Mg2+ is not important for structure and indicate that the main role of Mg2+ is to mediate optimization of active site chemistry. The Protein Journal, 29, 26–31.
Kurachi, M., & Aihara, H. I. (1984). Effect of a muscle relaxant, chlorphenesin carbamate, on the spinal neurons of rats. Japanese Journal of Pharmacology, 36, 7–13.
Yazar, K., Johnsson, S., Lind, M.-L., Boman, A., & Lidén, C. (2011). Preservatives and fragrances in selected consumer-available cosmetics and detergents. Contact Dermatitis, 64, 265–272.
Brown, V. L., & Orton, D. I. (2005). Two cases of facial dermatitis due to chlorphenesin in cosmetics. Contact Dermatitis, 52, 48–49.
Wakelin, S. H., & White, I. R. (1997). Dermatitis from chlorphenesin in a facial cosmetic. Contact Dermatitis, 37, 138–139.
Bridiau, N., Taboubi, S., Marzouki, N., Legoy, M. D., & Maugard, T. (2006). β-Galactosidase catalyzed selective galactosylation of aromatic compounds. Biotechnology Progress, 22, 326–330.
Scheckermann, C., Wagner, F., & Fischer, L. (1997). Galactosylation of antibiotics using the β-galactosidase from Aspergillus oryae. Enzyme and Microbial Technology, 20, 629–634.
Quan, J., Chen, Z., Han, C., & Lin, X. (2007). The synthesis of amphipathic prodrugs of 1,2-diol drugs with saccharide conjugates by high regioselective enzymatic protocol. Bioorganic & Medicinal Chemistry, 15, 1741–1748.
Quan, J., Xu, J.-M., Liu, B.-K., Zheng, C.-Z., & Lin, X.-F. (2007). Synthesis and characterization of drug–saccharide conjugates by enzymatic strategy in organic media. Enzyme and Microbial Technology, 41, 756–763.
Quan, J., Wu, Q., Zhu, L.-M., & Lin, X.-F. (2008). Chemo-enzymatic synthesis and sustained release of optically active polymeric prodrugs of chlorphenesin. Polymer, 49, 3444–3449.
Quan, J., Wu, Q., & Lin, X.-F. (2007). Synthesis of polymeric prodrugs of chlorphenesin with saccharide branches by chemo-enzymatic regioselective strategy. Polymer, 48, 2595–2604.
Jung, K.-H., Yeon, J.-H., Moon, S.-K., & Choi, J. H. (2008). Methyl α-D-glucopyranoside enhances the enzymatic activity of recombinant β-galactosidase inclusion bodies in the araBAD promoter system of Escherichia coli. Journal of Industrial Microbiology and Biotechnology, 35, 695–701.
Wiegand, I., Hilpert, K., & Hancock, R. E. W. (2008). Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature Protocols, 3, 163–175.
Yeon, J.-H., & Jung, K.-H. (2010). Change in compactness of inclusion bodies of recombinant β-galactosidase expressed in the araBAD promoter system of Escherichia coli. Biotechnology and Bioprocess Engineering, 15, 620–625.
Pfaller, M. A., Messer, S. A., Hollis, R. J., Jones, R. N., & the Sentry Participants Group. (2002). Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from sentry antimicrobial surveillance program. Antimicrobial Agents and Chemotherapy, 46, 1032–1037.
Rodríguez-Tudela, J. L., Berenguer, J., Martínez-Suárez, J. V., & Sanchez, R. (1996). Comparison of a spectrophotometric microdilution method with RPMI–2 % glucose with the national committee for clinical laboratory standards reference macrodilution method M27-P for in vitro susceptibility testing of amphotericin B, flucytosine, and fluconazole against Candida albicans. Antimicrobial Agents and Chemotherapy, 40, 1998–2003.
Andrews, J. M. (2001). Determination of minimal inhibitory concentrations. Journal of Antimicrobial Chemotherapy, 48(Suppl. S1), 5–16.
Anizon, F., Belin, L., Moreau, P., Sancelme, M., Voldoire, A., Prudhomme, M., et al. (1997). Syntheses and biological activities (topoisomerase inhibition and antitumor and antimicrobial properties) of rebeccamycin analogues bearing modified sugar moieties and substituted on the imide nitrogen with a methyl group. Journal of Medicinal Chemistry, 40, 3456–3465.
Pereira, E. R., Belin, L., Sancelme, M., Prudhomme, M., Ollier, M., Rapp, M., et al. (1996). Structure-activity relationships in a series of substituted indolocarbazoles: topoisomerase I and protein kinase C inhibition and antitumoral and antimicrobial properties. Journal of Medicinal Chemistry, 39, 4471–4477.
Cooper, R. D., Snyder, N. J., Zweifel, M. J., Staszak, M. A., Wilkie, S. C., Nicas, T. I., et al. (1996). Reductive alkylation of glycopeptide antibiotics: synthesis and antibacterial activity. Journal of Antibiotics (Tokyo), 49, 575–581.
Kahne, D., Leimkuhler, C., Lu, W., & Walsh, C. (2005). Glycopeptide and lipoglycopeptide antibiotics. Chemical Reviews, 105, 425–448.
Acknowledgement
The research was supported by a grant from the Academic Research Program of Korea National University of Transportation in 2013.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sang-Eun Lee, Tae-Min Jo, and Hyang-Yeol Lee contributed equally to this work and should be considered as equal first authors.
Korea National University of Transportation is formerly Chungju National University.
Electronic Supplementary Material
ESM 1
(DOCX 472 kb)
Rights and permissions
About this article
Cite this article
Lee, SE., Jo, TM., Lee, HY. et al. β-Galactosidase-Catalyzed Synthesis of Galactosyl Chlorphenesin and Its Characterization. Appl Biochem Biotechnol 171, 1299–1312 (2013). https://doi.org/10.1007/s12010-013-0213-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0213-3