Abstract
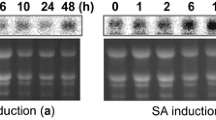
To identify novel components of basal resistance against the Tellitia indica of wheat, breeding for disease resistance was carried out on resistant and susceptible genotype of Karnal Bunt. The different members of wheat cystatin gene families were cloned, and their role in triggering differential resistance through co-expression was analyzed in our lab. The multidomain wheat cystatin (WCM) is a proteinase inhibitor characterized by cloning the gene from susceptible (WH542) and resistant genotype (HD 29). A WCM cDNA was isolated from both genotypes and sequenced. The WCM had a highly conserved N-terminal cystatin domain and a long C-terminal extension containing a second region, which exhibited similarity to the cystatin domain. The expression level was significantly (P > 0.001) higher in resistant compared to susceptible genotype at all the physiological stages of wheat spikes. In order to characterize the biochemical properties of WCM, the coding sequence was expressed in Escherichia coli using pET expression vector. The recombinant WCM was purified from soluble fraction of the cell extract by using affinity chromatography. WCM, with 23 KDa molecular mass, showed cysteine proteinase inhibitory activity against papain (Ki 3.039 × 10−7 M) as determined by using BAPNA as substrate. Furthermore, it was able to arrest the fungal mycelial growth of T. indica. Hyphae growth was inhibited, and morphological changes such as swelling and fragmentation of the fungus were observed. Overall, these observations suggest an endogenous high expression of cystatin, possibly associated with the resistance of wheat against Karnal bunt.





Similar content being viewed by others
Reference
Orlando, B.-H. (2004). Basic insight in plant–pathogen interaction. Biotecnologia Aplicada, 2(21), 1.
Kumar, J., & Nagarajan, S. (1998). Role of flag leaf and spike emergence stage on the incidence of Karnal bunt in wheat. Plant Disease, 82, 1368–1370.
Aujla, S. S., Grewal, A. S., Gill, K. S., & Sharma, I. (1989). A screening technique for Karnal bunt disease of wheat. Crop Improvement, 7, 145–146.
Rush, C. M., Stein, J. M., Bowden, R. L., Riemenschneider, R., Boratynski, T., & Royer, M. H. (2005). Status of Karnal bunt of wheat in the United States 1996 to 2004. Plant Disease, 89(3), 212–223.
Fuentes-Davila, G., & Rajaram, S. (1994). Sources of resistance to Tilletia indica in wheat. Crop Protection, 13(1), 20–24.
Bonde, M. R., Peterson, G. L., Fuentes, D. G., Aujla, S., Nanda, G. S., & Phillips, J. G. (1996). Comparison of the virulence of isolates of Tilletia indica, causal agent of Karnal bunt of wheat, from India, Pakistan, and Mexico. Plant Disease, 80, 1071–1074.
Bonde, M. R., Peterson, G. L., Schaad, N. W., & Smilanik, J. L. (1997). Karnal bunt of wheat. Plant Disease, 81, 1370–1377.
Singh, D. P., Singh, L. B., Kumar, X. J., Sinha, V. C., Singh, R. K., Sharma, I., et al. (2003). Post harvest surveys of wheat grains for the presence of Karnal bunt and black point diseases in different agroclimatic zones of India. Indian Journal of Agricultural Research, 37(4), 264–268.
Sansford, C. E., Baker, R. H. A., Brennan, J. P., Ewert, F., Gioli, B. A., Inman, A., et al. (2008). The new Pest Risk Analysis for Tilletia indica, the cause of Karnal bunt of wheat, continues to support the quarantine status of the pathogen in Europe. Journal of Plant Pathology, 57(4), 603–611.
Abe, K., Emori, Y., Kondo, H., Suzuki, K., & Arai, S. (1987). Molecular cloning of a cysteine proteinase inhibitor of rice (oryzacystatin). Homology with animal cystatins and transient expression in the ripening process of rice seeds. The Journal of Biological Chemistry, 262, 16793–16797.
Margis, R., Reis, E. M., & Villeret, V. (1998). Structural and phylogenetic relationships among plant and animal cystatins. Archives of Biochemistry and Biophysics, 359, 24–30.
Martinez, M., Abraham, Z., Gambardella, M., Echaide, M., Carbonero, P., & Diaz, I. (2005). The strawberry gene Cyf1 encodes a phytocystatins with antifungal activity. Journal of Experimental Botany, 56, 1821–1829.
Martinez, M., Cambra, I., Carrillo, L., Diaz-Mendoza, M., & Diaz, I. (2009). Characterization of the entire cystatin gene family in barley and their target cathepsin L-like cysteine-proteases, partners in the hordein mobilization during seed germination. Plant Physiology, 15(3), 1531–1545.
Silvia, V. R., Alberto, C. T., Victor, A. H., Erick, C. O., Alejandro, B. L., & Armando, G. R. (2010). Recombinant amaranth cystatin (AhCPI) inhibits the growth of phytopathogenic fungi. Plant Physiology and Biochemistry, 48, 469–475.
Yamada, T., Ohta, H., Shinohara, A., Iwamatsu, A., Shimada, H., Tsuchiya, T., et al. (2000). A cysteine protease from maize isolated in a complex with cystatin. Plant & Cell Physiology, 41, 185–191.
Alexandrov, N. N., Brover, V. V., Freidin, S., Troukhan, M. E., Tatarinova, T. V., Zhang, H., et al. (2009). Insights into corn genes derived from large-scale cDNA sequencing. Plant Molecular Biology, 69(1–2), 179–194.
Misaka, T., Kuroda, M., Iwabuchi, K., Abe, K., & Arai, S. (1996). Soyacystatin, a novel cysteine proteinase inhibitor in soybean, is distinct in protein structure and gene organization from other cystatins of animal and plant origin. European Journal of Biochemistry, 240, 609–614.
Abe, K., Kondo, H., Watanabe, H., Emori, Y., & Arai, S. (1991). Oryzacystatins as the first well-defined cystatins of plant origin and their target proteinases in rice seeds. Biomedica Biochimica Acta, 50, 637–641.
Valdes, R. S., Guerrero, R. A., Melgoza, V. C., Chagolla, L., Delgado, V. F., Martinez, G. N., et al. (2007). Cloning of a cDNA encoding a cystatin from grain amaranth (Amaranthus hypochondriacus) showing a tissue-specific expression that is modified by germination and abiotic stress. Plant Physiology and Biochemistry, 45, 790–798.
Waldron, C., Wegrich, L. M., Merlo, P. A., & Walsh, T. A. (1993). Characterization of a genomic sequence coding for potato multicystatin, an eight-domain cysteine proteinase inhibitor. Plant Molecular Biology, 23, 801–812.
Kimura, M., Ikeda, T., Fukumoto, D., Yamasaki, N., & Yonekura, M. (1995). Primary structure of a cysteine proteinase inhibitor from the fruit of avocado (Persea americana Mill). Bioscience, Biotechnology, and Biochemistry, 59, 2328–2329.
Lim, C. O., Lee, S. I., Chung, W. S., Park, S. H., Hwang, I., & Cho, M. J. (1996). Characterization of a cDNA encoding a cysteine proteinase inhibitor from Chinese cabbage (Brassica campestris L. ssp. pekinensis) flower buds. Plant Molecular Biology, 30, 373–379.
Pernas, M., Sanchez, M. R., & Salcedo, G. (2000). Biotic and abiotic stress can induce cystatin expression in chestnut. FEBS Letter, 467, 206–210.
Masonneau, A., Condamine, P., Wisniewski, J. P., Zivy, M., & Rogowsky, P. M. (2005). Maize cystatins respond to developmental cues, cold stress and drought. Biomedical Biochimica Acta, 1729, 186–199.
Christova, P. K., Christov, N. K., & Imai, R. (2006). A cold inducible multidomain cystatin from winter wheat inhibits growth of the snow mold fungus, Microdochium nivale. Planta, 223(6), 1207–1218.
Fernandes, K. V. S., Campos, F. A. P., Do Val, R. R., & Xavier-Filho, J. (1991). The expression of papain inhibitors during development of cowpea seeds. Plant Science, 74, 179–184.
Purwar, S., Marla, S. S., Singh, U. S., & Kumar, A. (2010). Basal expression studies of cystatins during specific growth stages of wheat spikes for defining their possible role in differential and stage dependent immunity against Karnal bunt (Tilletia indica). Molcular Biology Reports, 37, 1377–1389.
Ling, M., & Lewis, F. (2010). A rapid TRIzol-based two-step method for DNA-free RNA extraction from Arabidopsis siliques and dry seeds. Biotechnology Journal, 5(2), 183–186.
Thompson, J. D., Higgins, D. G., & Gibson, T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Research, 22, 4673–4680.
Tamura, K., Joel, D., Nei, M., & Kumar, S. (2007). MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4.0. Molecular Biology and Evolution, 24(8), 1596–1599.
Marchler-Bauer, A., Lu, S., Anderson, J. B., Chitsaz, F., Derbyshire, M. K., & DeWeese Scott, C. (2011). CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Research, 39(D), 225–229.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(7), 248–254.
Laemmli, U. K. (1970). Cleavage of structural proteins during assembly of the head of bacteriophages T4. Nature, 227, 680–685.
Wang, K. M., Kumar, S., Cheng, Y. S., Venkatagiri, S., Yang, A. H., & Yeh, K. W. (2008). Characterization of inhibitory mechanism and antifungal activity between group-1 and group-2 phytocystatins cystatin from taro (Colocasia esculenta). FEBS letter, 275, 4980–4989.
Kondo, H., Abe, K., Emori, Y., & Arai, S. (1991). Gene organization of oryzacystatin-II, a new cystatin superfamily member of plant origin, is closely related to that of oryzacystatin-I but different from those of animal cystatins. FEBS Letter, 278, 87–90.
Nagata, K., Kudo, N., Abe, K., Arai, S., & Tanokura, M. (2000). Three-dimensional solution structure of oryzacystatin-I, a cysteine proteinase inhibitor of the rice, Oryza sativa L. japonica. Journal of Biochemistry, 39(48), 14753–14760.
Rivard, D. L., Girard, C., Raphael, A., Vezina, P. L., Trpanier, S., & Michaud, D. (2007). MsCYS1, a developmentally-regulated cystatin from alfalfa. Plant Physiology and Biochemistry, 45, 508–514.
Kondo, H., Abe, K., Nishimura, I., Watanabe, H., Emori, Y., & Arai, S. (1990). Two distinct cystatin species in rice seeds with different specificities against cysteine proteinases. Journal Biological Chemistry, 265, 15832–15837.
Corre-Menguy, F., Cejudo, F. J., Mazubert, C., Vidal, J., Lelandais-Briere, C., Torres G., et al. (2002). Characterization of the expression of a wheat cystatin gene during caryopsis development. Plant Molecular Biology, 50, 687–698.
Abe, M., Abe, K., Kuroda, M., & Arai S. (1992). Corn kernel cysteine proteinase inhibitor as a novel cystatin superfamily member of plant origin. European Journal of Biochemistry, 209, 933–937.
Acknowledgments
I am highly thankful to the Department of Science and Technology for the financial support to complete this project. I am also thankful to Dr. Avinash Pandey, Coordinator, Nanotechnology Application Center, Allahabad University, Allahabad for providing the Scanning Electron Microscope facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Purwar, S., Sundaram, S., Verma, P. et al. A Physiologically Regulated Multidomain Cystatin of Wheat Shows Stage-Dependent Immunity Against Karnal Bunt (Tilletia indica). Appl Biochem Biotechnol 168, 2344–2357 (2012). https://doi.org/10.1007/s12010-012-9941-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9941-z