Abstract
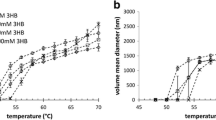
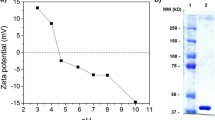
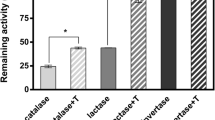
Yeast alcohol dehydrogenase (ADH) is an enzyme widely studied for biotechnological applications due to its involvement in fermentation industry, and various attempts to improve its catalytic properties and its thermal stability have been carried out. In this paper, the influence of a block copolymer (Poloxamer 407) on ADH enzymatic activity and thermal behaviour has been studied in order to get new insights about the use of poloxamers in formulation of sustained release systems for therapeutic proteins. Poloxamer 407 has the ability to form micelles and gel due to its self-assembling and thermoresponsive properties. The effect of the copolymer towards thermal stress and pH changes, which often reduce enzymes activity it has been investigated by means of enzymatic assays and differential scanning calorimetry. Results showed that at pH 9.1 and 7.3, the Poloxamer in the form of unimeric, micellar and gel state is able to effectively preserve the enzyme from thermoinactivation. In addition by calorimetric data Poloxamer 407 has showed an effect in preserving ADH from aggregation at pH 7.3. In conclusion, Poloxamer 407 seems to be very effective in protecting ADH from stress related events, like alkaline inactivation and aggregation.






Similar content being viewed by others
References
Dai, C., Wang, B., & Zhao, H. (2005). Microencapsulation peptide and protein drugs delivery system. Colloids and Surfaces. B, Biointerfaces, 41, 117–120.
Cleland, J. L., Daugherty, A., & Mrsny, R. (2001). Emerging protein delivery methods. Current Opinion in Biotechnology, 12, 212–219.
Bromberg, L. E., & Ron, E. S. (1998). Temperature-responsive gels and thermogelling polymer matrices for protein and peptide delivery. Advanced Drug Delivery Reviews, 31, 197–221.
Miyazaki, S., Takeuchi, S., Yokouchi, C., & Takada, M. (1984). Pluronic F-127 gels as a vehicle for topical administration of anticancer agents. Chemical and Pharmaceutical Bulletin, 32, 4205–4208.
Alexandridis, P., & Hatton, T. A. (1995). Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: thermodynamics, structure, dynamics, and modelling. Colloid Surf A-Physicochem Eng Asp, 96, 1–46.
Wang, P., & Johnston, T. P. (1991). Kinetics of sol-to-gel transition for poloxamer polyols. Journal of Applied Polymer Science, 43, 283–292.
Rassing, J., & Attwood, D. (1983). Ultrasonic velocity and light-scattering studies on the polyoxyethylene-polyoxypropylene copolymer Pluronic F127 in aqueous solution. International Journal of Pharmaceutics, 13, 47–55.
Vadnere, M., Amidon, G., Lindenbaum, S., & Haslam, J. L. (1984). Thermodynamic studies on the gel–sol transition of some pluronic polyols. International Journal of Pharmaceutics, 22, 207–218.
Wanka, G., Hoffmann, H., & Ulbricht, W. (1990). The aggregation behaviour of poly(oxyethylene)-poly(oxypropylene)-poly(oxyethylene) block copolymers in aqueous solution. Colloid & Polymer Science, 268, 101–117.
Pandit, N. K., & Wang, D. (1998). Salt effect on the diffusion and release rate of propanolol from poloxamer 407 gel. International Journal of Pharmaceutics, 167, 183–189.
Johnston, T. P., & Miller, S. C. (1985). Toxicological evaluation of poloxamers for intramuscular use. Journal of Parenteral Science and Technology, 39, 83–88.
Fults, K. A., & Johnston, T. P. (1990). Sustained release of urease from a Poloxamer gel matrix. Journal of Parenteral Science and Technology, 44, 58–65.
Johnston, T. P., Punjabi, M. A., & Froelich, C. S. (1992). Sustained release of interleukin-2 from a Poloxamer 407 gel matrix following intraperitoneal injection in mice. Pharmaceutical Research, 9, 425–434.
Pec, E. A., Wout, Z. G., & Johnston, T. P. (1992). Biological activity of urease formulated in Poloxamer 407 following intraperitoneal injection in the rat. Journal of Pharmaceutical Sciences, 81, 626–630.
Wang, P., & Johnston, T. P. (1995). Sustained-release interleukin-2 following intramuscular injection in rats. International Journal of Pharmaceutics, 113, 73–81.
Park, T. G., Cohen, S., & Langer, R. (1992). Poly(L-lactic acid)/Pluronic blends. Characterization of phase separation behaviour, degradation and morphology and use as protein-releasing matrices. Macromolecules, 25, 116–122.
Park, S. Y., Chung, H. J., Lee, Y., & Park, T. G. (2008). Injectable and sustained delivery of human growth hormone using chemically modified Pluronic copolymer hydrogels. Biotechnology Journal, 3, 669–675.
Chung, H. J., Lee, Y., & Park, T. G. (2008). Thermo-sensitive and biodegradable hydrogels based on stereocomplexed Pluronic multi-block copolymers for controlled protein delivery. Journal of Controlled Release, 127, 22–30.
Katakam, M., Bell, L. N., & Banga, A. K. (1995). Effect of surfactants on the physical stability of recombinant human growth hormone. Journal of Pharmaceutical Sciences, 84, 713–716.
Katakam, M., Ravis, W. R., & Banga, A. K. (1997). Controlled release of human growth hormone in rats following parenteral administration of poloxamer gel. Journal of Controlled Release, 49, 21–26.
Williams, R. J. P. (1995). Energised (entatic) states of groups and of secondary structures in proteins and metalloproteins. European Journal of Biochemistry, 234, 363–381.
Miroliaei, M., Ranjbar, B., Naderi-Manesh, H., & Nemat-Gorgani, M. (2007). Thermal denaturation of yeast alcohol dehydrogenase and protection of secondary and tertiary structural changes by sugars: CD and fluorescence studies. Enzyme and Microbial Technology, 40, 896–901.
Yang, Y., & Zhou, H. M. (2001). Effect of zinc ions on conformational stability of yeast alcohol dehydrogenase. Biochemistry (Mosc), 66, 47–54.
Arakawa, T., & Timasheff, S. N. (1985). The stabilization of proteins by osmolytes. Biophysical Journal, 47, 411–414.
Timasheff, S. N. (2002). Protein-solvent preferential interactions, protein hydration, and the modulation of biochemical reactions by solvent components. PNAS, 99, 9721–9726.
Auton, M., Bolen, D. W., & Rösgen, J. (2008). Structural thermodynamics of protein preferential solvation: osmolyte solvation of proteins, aminoacids, and peptides. PROTEINS: Structure, Function and Bioinformatics, 73, 802–813.
Foreman, T. M., Khalil, M., Meier, P., Brainard, J. R., Vanderberg, L. A., & Sauer, N. N. (2001). Effects of charged water-soluble polymers on the stability and activity of yeast alcohol dehydrogenase and subtilisin carlsberg. Biotechnology and Bioengineering, 76, 241–246.
Markossian, K. A., Golub, N. V., Khanova, H. A., Levitsky, D. I., Poliansky, N. B., Muranov, K. O., et al. (2008). Mechanism of thermal aggregation of yeast alcohol dehydrogenase I—role of intramolecular chaperone. BBA, 1784, 1286–1293.
Vanni, A., Anfossi, L., Pessione, E., & Giovannoli, C. (2002). Catalytic and spectroscopic characterisation of a copper-substituted alcohol dehydrogenase from yeast. International Journal of Biological Macromolecules, 30, 41–45.
Barzegar, A., Moosavi-Movahedi, A. A., Kyani, A., Goliaei, B., Ahmadian, S., & Sheibani, N. (2010). New model for polymerization of oligomeric alcohol dehydrogenases into nanoaggregates. Applied Biochemistry and Biotechnology, 160, 1188–1205.
Sanchez-Ruiz, J. M. (1992). Theoretical analysis of Lumry–Eyring models in differential scanning calorimetry. Biophysical Journal, 61, 921–935.
Bergmeyer, H. U. (1974). Methods of enzymatic analysis (Bergmeyer H.U. Editions) Verlag Chemie, Weinheim and Academic Press, New York.
Segel, I. H. (1975). Enzyme kinetics—behavior and analysis of rapid equilibrium and steady-state enzyme systems (pp. 100–160). NY: Wiley.
Bonacucina, G., Spina, M., Misici-Falzi, M., Cespi, M., Pucciarelli, S., Angeletti, M., et al. (2007). Effect of hydroxypropyl β-cyclodextrin on the self-assembling and thermogelation properties of Poloxamer 407. European Journal of Pharmaceutical Sciences, 32, 115–122.
Meredith, S. C. (2005). Protein denaturation and aggregation. cellular responses to denatured and aggregated proteins. Annals of the New York Academy of Sciences, 1066, 181–221.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pucciarelli, S., Bonacucina, G., Bernabucci, F. et al. A Study on the Stability and Enzymatic Activity of Yeast Alcohol Dehydrogenase in Presence of the Self-Assembling Block Copolymer Poloxamer 407. Appl Biochem Biotechnol 167, 298–313 (2012). https://doi.org/10.1007/s12010-012-9692-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9692-x