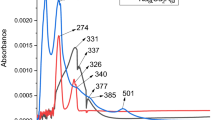
Abstract
Four hydroxyflavone derivatives have been synthesized with the aim of obtaining a good model of superoxide dismutase. Better to mimic the natural metalloenzyme, copper complexes have been designed. The Cu(II) complexes of general formulae, [CuL] where L = 5-hydroxyflavone-o-phenylenediamine (L1H2)/m-phenylenediamine (L2H2) and 3-hydroxyflavone-o-phenylenediamine (L3H2)/m-phenylenediamine (L4H2) have been synthesized. The structural features have been determined from their analytical and spectral data. All the Cu(II) complexes exhibit square planar geometry. Redox behavior of copper complexes was studied and the present ligand systems stabilize the unusual oxidation state of the copper ion during electrolysis. The in vitro antimicrobial activities of the investigated compounds were tested against the bacterial species Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Proteus vulgaris, and Pseudomonas aeruginosa and fungal species Aspergillus niger, Rhizopus stolonifer, Aspergillus flavus, Rhizoctonia bataicola, and Candida albicans. Superoxide dismutase and anti-inflammatory activities of the copper complexes have also been measured and discussed.




Similar content being viewed by others
References
Yamada, S. (1999). Coordination Chemistry Reviews, 192, 537.
Jeewoth, T., Bhowon, M. G., & Wah, H. L. K. (1999). Transition Metal Chemistry, 24, 445.
Saczewski, F., Dziemidowicz-Borys, E., Bednavski, P. J., Grunert, R., Gdaniec, M., & Tabin, P. (2006). Journal of Inorganic Biochemistry, 100, 1389.
Anacona, J. R., Rodriguez, C., & Rodriguez-Barbarin, M. C. (2004). Monatshefte fur Chemie, 135, 785.
Anacona, J. R., Nusetti, O., Gutierrez, C., & Loroño, D. (2002). Journal of Coordination Chemistry, 55, 1433.
Guinovart, C., Navia, M. M., & Tanner, M. (2006). Current Molecular Medicine, 6, 137.
Rice-Evans, C. (2001). Current Medicinal Chemistry, 8, 797.
Swearingen, J. K., & West, D. X. (2001). Transition Metal Chemistry, 26, 252.
Sorenson, J. R. J. (1996). Journal of Medicinal Chemistry, 19, 135.
Vogel, A. I. (1969). Text book of quantitative inorganic analysis (7th edition). London.
Looker, L. H., Edman, J. R., & Dappen, J. I. (1964). Journal of Heterocyclic Chemistry, 1, 141.
Arudi, R. L., Allen, A. O., & Bielski, B. H. J. (1981). FEBS Letters, 135, 265.
Pereira, R. M., Andrades, N. E., Paulino, N., Sawaya, A. C., Eberlin, M. N., Marcucci, M. C., et al. (2007). Molecules, 12(7), 1352–1366.
Geary, W. J. (1971). Coordination Chemistry Reviews, 7, 81–122.
Nakamoto, K. (1988). Spectroscopy and structure of metal chelate compounds (p. 214). New York: Wiley.
Mostahar, S., Alam, S., & Islam, A. (2006). Indian Journal of Chemistry, 45B, 1478–1486.
Sarkar, S., Dhara, P. K., Nethaji, M., & Chattopadhyay, P. (2009). Journal of Coordination Chemistry, 62, 817–824.
Li, T. R., Yang, Z. Y., Wang, B. D., & Qin, D. D. (2008). European Journal of Medicinal Chemistry, 43, 1688.
Chandra, S., & Gupta, L. K. (2005). Spectrochimica Acta A, 61, 269.
Gaballa, A. S., Asker, M. S., Barakat, A. S., & Teleb, S. M. (2007). Spectrochimica Acta A, 67, 114–121.
Diaz, A., Cao, R., Fargoso, A., & Sanchez, I. (1999). Inorganic Chemistry Communications, 2, 358.
Patole, J., Dutta, S., Padhye, S., & Sinn, E. (2001). Inorganica Chimica Acta, 318, 207–211.
Anjaneyula, Y., & Rao, R. P. (1986). Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry, 16, 257–272.
Anacona, J. R., Lorono, D., Azocar, M., & Atencio, R. (2009). Journal of Coordination Chemistry, 62, 951.
Levinson, W., & Jawetz, E. (1996). Medical microbiology and immunology, 4th Ed. Stanford.
Mishra, L., & Singh, V. K. (1997). Indian Journal of Chemistry Section A, 32, 446.
Satoskar, R. S., & Bhandarkar, S. D. (1933). Pharmacology and Pharmacotherapeutics (13th ed., p. 252). Bombay: Popular Prakash Pvt Ltd.
Bhirud, R. G., & Srivastava, T. S. (1990). Inorganica Chimica Acta, 173, 121.
Blahova, M., Tumova, I., Sokolik, J., Orosova, I., & Svec, P. (1998). Ceská a Slovenská Farmacie, 47, 166–9.
Zhiqiang, S., Lei, W. Y., Li, L., Chen, Z. H., & Liu, W. P. (1998). Inflammopharmacology, 6, 357–362.
Acknowledgments
The authors express their sincere thanks to the Chairman, Noorul Islam University for his constant encouragement and providing research facilities. The authors gratefully acknowledge DST, New Delhi for financial support under INSPIRE fellowship (IF 10544).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joseph, J., Nagashri, K. Novel Copper-Based Therapeutic Agent for Anti-Inflammatory: Synthesis, Characterization, and Biochemical Activities of Copper(II) Complexes of Hydroxyflavone Schiff Bases. Appl Biochem Biotechnol 167, 1446–1458 (2012). https://doi.org/10.1007/s12010-011-9529-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9529-z