Abstract
A novel mannan-specific lectin was isolated from the roots of a traditional Chinese herbal medicine, Ophioglossum pedunculosum through ion-exchange chromatography and gel filtration. With a molecular mass of 19,835.7 Da demonstrated by MALDI-TOF analysis, this novel agglutinin was designated as O. pedunculosum agglutinin (OPA), specifically agglutinating human O erythrocytes and rabbit erythrocytes. The hemagglutination could be strongly inhibited by mannan and thyroglobulin, the activity of which was stable in pH range of 4.0–8.0 and at temperatures below 50 °C. Chemical modification studies indicated that tryptophan and arginine residues were essential for its hemagglutinating activity. Meanwhile, it showed antifungal activities toward Sclerotium rolfsii and Fusarium graminearum. In addition, to amplify cDNA of OPA by 3′/5′-rapid amplification of cDNA ends (RACE), the N-terminal 30 amino acids sequence of OPA was determined, and degenerate primers were designed. The obtained full-length cDNA of OPA contained 885 bp with an open-reading frame of 600 bp encoding a precursor protein of 199 amino acids, while the mature protein had 170 amino acids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Distributed in plants, animals, fungi, bacteria, and viruses [1–4], lectins are carbohydrate-binding proteins or glycoproteins of nonimmune origin that specifically and reversibly bind to carbohydrates of glycoconjugates [5–7]. According to serological relationships, sequence similarities, and their evolutionary relationships, plant lectins have been classified into 12 families as follows [8]: Agaricus bisporus agglutinin (ABA), Amaranthin, chitinase-related agglutinin (CRA), Cyanovirin, Euonymus europaeus (EEA agglutinin), Galanthus nivalis agglutinin (GNA), Hevein, Jacalins, legume lectin, lysine motif (LysM), Nictaba, and Ricin B families. The lectins mentioned above possess a variety of biological functions including antitumor, immunomodulatory, antifungal, antivirus, and antiinsect activities [9–19].
Obtained from plant sources, lectins have been extensively studied. However, the inventory of carbohydrate-binding domains in plant cells is still incomplete, and very little information is available on lectins of ferns [8]. As for ferns, there are a large number of reports regarding the paleobotanical, morphological, and molecular studies such as Marattiaceae, Ophioglossum L., Adiantum L., etc. [20]. Even all plant lectins can be virtually classified into existing protein families, exceptions still exist. One of exceptions is Ophioglossum, one genera of Ophioglossaceae is commonly called the adder’s tongue ferns, a putatively ancient lineage of pteridophytes with an evolutionary history [21]. It is nearly cosmopolitan, playing an important role in medicine [22]. Ophioglossum is a traditional Chinese medicinal herb and possesses many health-promoting and therapeutic effects such as cough and antigastric ulcer activity [23].
In this study, we reported the purification of a novel lectin from roots of Ophioglossum pedunculosum, as well as cDNA cloning and characterization. We also compared its physicochemical and biological characteristics with other fern lectins to investigate characteristics and potentially exploitable activities of O. pedunculosum lectin. Furthermore, O. pedunculosum agglutinin (OPA) represents a novel lectin family that shared no significant sequence similarity with any other known lectin families. In a word, this study provided useful information, enriching the study of fern lectins and revealing the use in agriculture.
Materials and Methods
Plant Material
O. pedunculosum was obtained from Pengzhou, Sichuan, China. The herbarium deposit number in Sichuan University herbarium is 001436320.
Purification of Lectin
About 100 g roots of O. pedunculosum were homogenized in sodium chloride solution and soaked for 24 h at 4 °C. The mixture was then centrifuged at 5,900 × g for 25 min at 4 °C. The supernatant was subjected to 80% ammonium sulfate precipitation. Subsequent to centrifugation, the precipitate was dissolved in and dialyzed against 50 mM Tris-HCl buffer, pH 8.0. The crude extract was loaded on a column of DEAE-Sepharose (Sigma) previously equilibrated and eluted with 50 mM Tris-HCl buffer, pH 8.0. After washing of unadsorbed materials, the adsorbed fractions were eluted with 0–0.5 M NaCl gradient in Tris-HCl buffer. Fractions with hemagglutinating activity (HA) were pooled and dialysed against 20 mM PBS, pH 7.0. After concentrated, the samples were subjected to a column of Sephacryl S-100 in 20 mM PBS, pH 7.0.
Determination of Molecular Mass
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was used to estimate the subunit molecular mass of OPA as described by Laemmli [24]. The native molecular mass was estimated by gel filtration on the Sephacryl S-100 column, according to the method of Andrew [25].
The more precise molecular mass of OPA was determined by matrix-assisted laser desorption/ionization time-of-flight (MALDI–TOF) mass spectrum (Bruker Autoflex II) according to the method of Laremore et al. [26].
Determination of N-terminal Sequence
The N-terminal sequence of the lectin polypeptides was carried out using a Hewlett-Packard (HP) G1000A Edman degradation unit and an HP 1000 high-performance liquid chromatography system [27].
Hemagglutinating Activity
The HA was assessed using human A, B, AB, O, and rabbit erythrocytes. HA assays were carried out in microtiter U-plate according to a twofold serial dilution procedure. The lectin solution (50 μl) was mixed with 50 μl of a 2% suspension of red blood cells in phosphate-buffered saline (PBS), pH 7.2 at 20 °C. The results were read after about 1 h when the blank had fully sedimented. The hemagglutination titer, defined as the reciprocal of the highest dilution exhibiting hemagglutination, was reckoned as one hemagglutination unit. Specific activity was the number of hemagglutination units per mg protein [18].
Carbohydrate-Binding Specificity
Serial twofold dilutions of sugar samples were prepared in PBS. The initial concentration of carbohydrates and glycoproteins was 100 mM and 4 mg/ml, respectively. All of the dilutions were mixed with an equal volume (25 μl) of a solution of the lectin with 32 hemagglutination units. The mixture was allowed to stand for 30 min at room temperature and then mixed with 50 μl of a 2% rabbit erythrocyte suspension. The minimum concentration of the sugar in the final reaction mixture, which completely inhibited 32 hemagglutination units of the lectin preparation, was calculated [18].
Effects of Temperature and pH on HA
The effects of temperature and pH on HA of the lectin were examined as previously described [18, 28].
Chemical Modification of OPA
Modification of Tryptophan Residue
Modification of tryptophan residues was carried out using N-bromosuccinimide (NBS) according to the method of Spande and Witkop [29]. The lectin solution (1.0 mg/ml) prepared in PBS buffer (pH 5.1) was divided into three aliquots. Aliquot 1 in the absence of NBS was served as control. Urea (8 M) was added into aliquot 3. NBS (6 μl, 10 mM) was titrated into aliquots 2 and 3 every 5 min. After every addition of NBS, an aliquot was removed and quenched with a 10 μl 10 mM tryptophan solution, and the residual activity was determined after removal of excess reagent by dialysis. The NBS-mediated lectin inactivation was also monitored using a spectrophotofluorometer (Model 4500, Hitachi, Tokyo, Japan) by measuring the decrease in fluorescence at 295 nm. The number of tryptophan residues oxidized was calculated as described by Spande and Witkop [29].
Modification of Histidine Residue
Modification of histidine residues was carried out by diethylpyrocarbonate (DEPC) followed the method described by Komath et al. [30]. The HA was assayed after the excessive reagent was removed by dialyzing against deionized water.
Modification of Arginine Residue
According to the method described by Laszlo and Smith [31], arginine residues were modified with 2,3-butanedione. After dialyzing against the deionized water, the HAs were determined.
Spectroscopic Measurements
All the fluorescence spectrum of the testing sample were made on spectrophotofluorometer (Model 4500, Hitachi, Tokyo, Japan) according to the previous method [32] using a 1 cm quartz cuvette at room temperature. The samples were excited at 280 and 295 nm, and the emission spectra were recorded ranging from 300 to 450 nm.
Assay of Antifungal Activity
The assay for antifungal activity was performed using Petri dishes (100 × 15 mm) containing 10 ml of potato dextrose agar. After the mycelial colony had developed, sterile blank paper disks (0.5 cm in diameter) were placed at a distance of 0.5 cm away from the rim of the mycelial colony. Then lectin solutions with different concentration were added to the disks. The Petri dishes were then incubated at 28 °C for 72 h until mycelial growth had enveloped disks containing the control and had formed crescents of inhibition around disks with antifungal samples [33, 34]. Three fungal species Sclerotium rolfsii, Fusarium graminearum, and Gibberella zeae were examined in the assay.
RNA Extraction and cDNA Cloning
Total cellular RNA was extracted using Trizol reagent (Invitrogen, USA) according to the manufacturer’s instructions from underground roots of O. pedunculosum. The RNA quality was confirmed by 1% agarose gel electrophoresis.
3′-Full rapid amplification of cDNA end (RACE) Core set ver 2.0 (TAKARA, China) was used to obtain OPA cDNA 3′-fragment, and all 3′-RACE steps were carried out according to the attached protocol. Two degenerate primers (Primer 1: 5′-GYACNCARGAYGAYAAYGAYCA-3′, Primer 2: 5′-TNTTYGGNACNGAYGCNCA-3′) were designed based on the sequence of the first sequenced 17 N-terminal amino acid residues of OPA. After reverse transcription with 3′-RACE adaptor, nested PCR was carried out. The cDNA population was used as template for the outer PCR reaction with the Primer 1 and 3′-RACE outer primer under the following cycling program: 94 °C for 5 min, 94 °C for 30 s, 53 °C for 30 s, 72 °C for 1 min and 30 cycles, 72 °C for 10 min. The outer PCR product was used as template in inner PCR with Primer 2 and 3′-RACE inner primer. The PCR product was analyzed on 1% agarose gel. The cDNA fragment with appropriate size (near to 900 bp) was recovered from the gel, cloned into pMD 18-T vector (TAKARA, China) and transformed to Escherichia. coli Top 10 F′. The positive single recombinant colonies were selected and sequenced. Based on the result, 5′-RACE-specific primers 3, 4, and 5 were synthesized (Primer 3: 5′-CATTGCTTGTACTTGGCTTC-3′, Primer 4: 5′-CTGCTTCTCCTCAAACCACT-3′, Primer 5: 5′-GAGCCTCTTCACGATTCTTT-3′). Then a homopolymeric tail (dA) was appended to the 3′ end of the purified cDNA with terminal deoxynucleotidyl transferase (TAKARA, China). Likewise, the cDNA 5′ end was obtained by 5′-RACE in essentially the same manner as that for the cDNA 3′ end.
After concatenation of the nucleotide sequence of the 3′- and 5′-RACE sequences, the amplification of full-length cDNA of OPA was acquired using Primer 6: 5′-AAAAGCAGAAGAAGGTAAAG −3′ and Primer 7: 5′-TAAAACCTTCACTGCAAAC-3′ under the action of pfu DNA polymerase (TAKARA, China).
Statistical Analysis of the Data
All the data were confirmed in at least three independent experiments. Statistical analyses were utilized using SPSS software.
Results
Purity Examination and Molecular Mass Determination
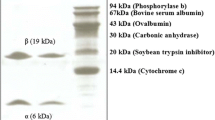
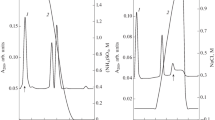
The dissolved (NH4)2SO4-precipitated fraction of the roots extract was fractionated on DEAE-Sepharose into a flow-through fraction A1 with HA and three adsorbed fractions A2, A3, and A4 without activity (Fig. 1a). A1 was subsequently fractionated on Sephacryl S-100 into two adsorbed fractions B1 and B2 (Fig. 1b). HA residing in fraction B2 corresponding to the main peak was collected and represented purified lectin. B2 appeared as a single band in SDS-PAGE (Fig. 2a), and a single peak in gel filtration on Sephacryl S-100 (Fig. 2b). The resulting protein product had a molecular mass of 19,835.7 Da identified by MALDI-TOF MS (Fig. 2c), which was consistent with its calculated value of 19.8 kDa by SDS-PAGE and gel filtration. The overall purification yield of the lectin was summarized in Table 1.
Purification of Ophioglossum pedunculosum agglutinin. a Anionic exchange chromatography of crude extract of O. pedunculosum roots on a DEAE-Sepharose column (1.6 × 20 cm2) in 0.05 M Tris-HCl buffer (pH 8.0) at a flow rate of 2 ml/min. The peaks labeled A1 exhibited HA. b Gel filtration of fraction A1 on a Sephacryl S-100 column in 0.02 M PBS (pH 7.0) at a flow rate of 0.4 ml/min. Arrows indicated the point at which buffer was changed. Detection of the optical density of each fraction was at A280
a 12% SDS-PAGE analysis of OPA. Lane 1: Molecular mass markers, from top downward: Phosphorylase b (94 kDa); albumin bovine (66.2 kDa); ovalbumin (45 kDa); carbonic anhydrase (35 kDa); trypsin inhibitor (21 kDa); and alactalbumin (14.4 kDa). Lane 2: In the presence of 2-mercaptoethanol. b Native molecular mass estimation of OPA on Sephacryl S-100 gel filtration chromatography column. Standards used for gel filtration analysis were soybean trypsin inhibitor (30.2 kDa), bovine serum albumin (67 kDa), ovalbumin (45 kDa), chymotrypsinogen A (25 kDa), and cytochrome C (12.5 kDa). c Molecular mass determination by MALDI-TOF MS
HA and Carbohydrate-Binding Specificity
The lectin OPA agglutinated specifically blood type O of human erythrocytes, but exhibited no agglutination towards other type of human erythrocytes (A, B, AB). The minimum amount of lectin to agglutinate normal human erythrocytes and rabbit erythrocytes were 3.90 and 1.95 μg/ml, respectively.
To enumerate the carbohydrate-binding specificity of OPA, hemagglutination inhibition assays were performed using a panel of sugars and glycoproteins. The results demonstrated that the HA of OPA was most potently inhibited by mannan and thyroglobulin (Table 2), while the IC50 values of mannan and thyroglobulin (required for 50% inhibition) were 12.5 mM and 2 mg/l, respectively. However, there was no inhibition by mannose, galactose, fucose, GlcNAc, glucose, maltose, lactose, fructose, and xylose.
Effects of Temperature and pH on HA of OPA
The effects of temperature and pH on HA of OPA were shown in Fig. 3a and b, respectively. The HA of OPA was stable up to 50 °C, while 10% of the activity was lost at 60 °C. However, there was still less than 20% activity maintained even at 100 °C for 20 min. OPA was stable in a broad pH range from pH 4.0–8.0, although its activity gradually disappeared when pH values were above 8.0 or below 3.0. Interestingly, OPA could maintain 50% activity at pH 2.0 whereas only 5% at pH 12.0, which indicated that it was more suitable for preserving OPA activity under acidic condition.
Effects of temperature and pH on HA of OPA. a Thermal stability of OPA. The lectin was incubated at an elevated temperature (20–100 °C). The line represented the percentage HA of OPA. b pH stability of OPA. The lectin was incubated with buffers ranging from pH 2.0 to 12.0. The line represented the percentage HA of OPA
Chemical Modification Studies
Various reagents were used to modify special amino acid residues in OPA. After treatment with NBS, a number of tryptophan residues modified by NBS versus remaining percentage residual activity of OPA indicated that three tryptophan residues were modified in native conditions, approximately 27% HA was lost after modification of the third Trp residues (data not shown). Number of tryptophans modified in urea denatured lectin was also three. NBS-mediated inactivation of the lectin was accompanied by decrease in fluorescence intensity of modified protein at 295 nm, while the concentration of NBS increased (Fig. 4). The number of modified Trp residues in the presence of mannan did not change. In addition, modification of Arg residues with 2, 3-butanedione resulted in 70% loss of the HA of the lectin, while modification of His residues with DEPC did not affect the HA of the lectin (Table 3).
Effect of modification of Trp on HA of OPA. Fluorescence spectra of OPA with different concentration of NBS. Curves from top to bottom represent the fluorescence spectra after the addition of 0, 4, 12, 18, 24, and 30 μl of 10 mM NBS. Excitation was carried out at 295 nm and the emission spectra were recorded between 300 and 400 nm
Assay of Antifungal Activity
The purified OPA showed antifungal activity against some fungal species. Especially, it strongly inhibited the growth of S. rolfsii and F. graminearum at the concentration of 40 and 70 μg/ml, separately (Fig. 5). However, the effect on G. zeae was undetectable, even at the concentration of 1 mg/ml.
Antifungal activity of OPA toward Fusarium graminearum and Sclerotium rolfsii in vitro. a F. graminearum. Different concentration of OPA (Primer 1. 560, μg/ml; Primer 2. 280 μg/ml; Primer 3. 140 μg/ml; 4. 70 μg/ml) in 0.1 M PBS buffer (pH 7). b S. rolfsii. Different concentration of OPA (Primer 1. 640 μg/ml; Primer 2. 320 μg/ml; 3. 160 μg/ml; 4. 80 μg/ml; 5. 40 μg/ml) in 0.1 M PBS buffer (pH 7)
N-terminal Sequence and cDNA Cloning of OPA
The N-terminal sequence of OPA was SCTQDDNDQRQLFGTDAHSSENEAKQDNVA. Therefore, the two degenerate primers were synthesized according to the underlined amino acids deduced to nucleotides. After 3′-RACE reaction with them, the amplification products showed two main bands in agarose electrophoresis (Fig. 6a). One band was about 500 bp and the other about 900 bp. According to the molecular mass, the 900 bp fragment band was recovered and sequenced. Sequentially, the amplification products of 5′-RACE reaction showed one band about 450 bp (Fig. 6b). Based on these results, cDNA sequence of OPA was obtained (Fig. 7), which encoded a precursor protein of 199 amino acids with a signal peptide of 29 amino acids and the cleaving site was between the 29th and 30th amino acids. What’s more, the calculated molecular mass of mature OPA (19,804.24 Da) (http://cn.expasy.org/cgi-bin/pi_tool) was in good agreement with the molecular mass of native OPA (19,835.7 Da) determined by MALDI-TOF.
Agarose electrophoresis results. All agarose electrophoreses in this study were carried out on 1% agarose gel. a Result of 3′-RACE. The amplification products showed two main bands with two degenerate primers. One band was about 500 bp and the other about 900 bp. According to the molecular mass, the 900 bp fragment band was recovered and sequenced. b Result of 5′-RACE. The amplified product was about 450 bp with three specific primers designed according to the 3′-RACE result
Nucleotide sequence of cDNA and deduced amino acid sequence for Ophioglossum pedunculosum Lectin. The first 29 amino acids was signal peptide of OPA. And red letters were the sequenced 30 amino acids. Two degenerate primers for 3′-RACE were designed based on the sequence of the underlined amino acid residues
Discussion
Although high levels of lectins were widely distributed into storage tissues (seeds, bark, rhizomes, bulbs, etc.) of flowering plants, low levels of lectins and related proteins in the so-called “primitive” vascular plants (ferns and fern allies) had been investigated only occasionally, such as lectins from Azolla-Anabaena symbiosis, nonsymbiotic Azolla (Salviniaceae), Azolla pinnata, Azolla filiculoides, Dryopteris (Polypodiaceae) ferns, Phlebodium aureum, Blechnum Orientale, Dryopteris championi, Woodwardia jatonica, Adiantum flabellulatum, and Ceratopteris richardii [35–42]. All of these genera are in the class of true ferns, Filicinae. To our knowledge, no other reports of lectins in true ferns or fern allies had been made. Thus, our report represented detailed investigation of a lectin from the plant division Pteridophyta. Results indicated that OPA was a monomer of 19,835.7 Da, similar with lectins from Dryopteris champion, Woodwardia jatonica, and Adiantum flabellulatum, which was also consistent with the previously published biochemical features for other plant lectins within the 17 to 20 kDa range [43, 44].
It was mentioning that OPA specifically agglutinated human O erythrocytes and rabbit erythrocytes, suggesting that OPA recognized the structure of saccharides comprising the surface of erythrocyte membranes. A similar result was demonstrated for Dryopteris championi lectin with specificity on animal species and human blood group [39], while the lectins from Adiantum flabellulatum [41] and Woodwardia jatonica [40] agglutinated both rabbit erythrocytes and human erythrocytes with no specificity. Agglutination occurred on the premise that the lectin must bind to the surface of the erythrocytes and formed a cross-bridge between them. There was, nevertheless, no simple relationship between the amount of lectin-bound and agglutination. Many factors had a great influence on agglutinating activities such as the sort of the carbohydrate and the accessibility of receptor sites. Besides, the external conditions of temperature, cell concentration, and mixing were also important elements. But this difference in the agglutination ability of OPA could be mainly owing to the nature of specific carbohydrate-binding sites on the cell surfaces recognized weakly or not totally by the lectin.
In order to further identify the carbohydrate-binding specificity, we performed the carbohydrate inhibition analyses. The results demonstrated that OPA was distinctive in that its HA could not be inhibited by a variety of simple sugars except thyroglobulin and mannan. However, most fern lectins were inhibited by monosaccharides, or their derivates, or monosaccharides and glycoprotein, such as Phlebodium aureum lectin, Woodwardia jatonica lectin, Blechnum Orientale lectin, Dryopteris championi lectin, Azolla–Anabaena lectin, Azolla pinnata lectin, and Azolla filiculoides lectin [35–40]. As we know, few purified fern lectins could be inhibited both by oligosaccharides and glycoprotein to date. In this study, we found that OPA was endowed this activity. This inhibitory profile was similar to some aroid lectins such as A. maculatum lectin, C. esculenta lectin, and Xanthosoma sagittifolium lectin, which were also precipitated by both asialoglycoproteins and yeast mannan [45]. These results indicated that OPA could possess two distinctly different types of carbohydrate-combining sites, and it maybe a special mannan-binding lectin or might represent the first member of a new lectin family.
Results of chemical modification by analysis of HA and fluorescence alterations provided a useful clue for the identification of amino acid residues present in or near the functional or active site of lectins [46]. Treating purified OPA with NBS, a reagent specific for Trp residues in restricted conditions, resulted in a 27% drop in the activity of OPA, which suggested the partial necessity of Trp residues for the activity of OPA. It might be located on surface or the shallow grooves of the molecule since tryptophan could be modified completely without denaturant [47]. It was an evident that an obvious decrease and blue shift (from 340 nm to 330 nm) of the fluorescence emission spectrum were observed with the increase of NBS concentration, which showed that the tryptophan residue of OPA located in hydrophobic areas on the surface of the molecule or the indole rings of tryptophan residue might be in special conformation [48]. The presence of the haptenic sugar mannan in the assay medium did not provide protection for OPA against NBS inactivation, which indicated that tryptophan might not be present in the lectin affinity site. Modification of arginine residue also showed partial necessity of Arg residue in the activity of OPA; aspartic acid or glutamic acid residues might be involved in maintaining the crucial conformation of activity center, and making great contribution to the HA of OPA molecule directly (data not shown). However, His residues did not cause any change in the HA of OPA and the result ruled out the possibility that histidine residues were involved in the HAs of OPA.
Antifungal lectins had been drawing the attention of many researchers because of their ability to deter pathogenic fungi from invading agricultural crops and causing diseases in animals. Hitherto, only a few lectins had been reported to possess remarkable antifungal activity [49], only two from ferns: Woodwardia japonica lectin inhibited the growth Helminthosporium turcicum and Pseudomonas solanacoarum while Blechnum Orientale lectin inhibited the growth of Helminthosporium turcicum [40]. Interestingly, in the present study, OPA exhibited potent antifungal activity towards two commonly agriculturally harmful fungi, S. rolfsii and F. graminearum, suggesting OPA might play an important role against fungal infections in plants. The detailed mechanism of inhibition the growth of fungi by lectins is unclear. Lectin from Adiantum flabellu latum had no antifungal activity [41], although it had some resemblance of homogeneity and molecular mass, dependence of temperature and pH, even the sequence of amino acid comparing with Woodwardia japonica lectin. It should be explained in terms of their carbohydrate-binding specificity and structure. Mannan, chitin and other saccharides were important components of most fungal cell walls. OPA, as a monomer with relatively small mass, could bind to the glycoprotein or mannans of the fungal cell wall and ultimately altered the normal cross-linking activities during cell wall formation, severely affecting the growth and pathogenicity of the fungus [43, 50, 51]. Moreover, the ability of OPA to inhibit fungal growth differs among fungal species should be owing to the different recognized sites and the characteristics of fungi. However, direct evidence of an interaction between OPA and cellular components was necessary in order to unravel the mechanisms of action of this protein.
More importantly, we described a successful procedure for the cloning of OPA cDNA. To our knowledge, the full-length sequences of true ferns, had been reported PAL [37] and Ceratopteris richardii lectin [42] only. Here, we reported another cDNA sequence of a true fern lectin, OPA. Since the degenerate primers were synthesized according to the first 17 N-terminal amino acids of the sequenced 30 ones. Naturally, the following 13 amino acids sequence coincided with the translation of corresponding cDNA fragment suggesting the correctness of cDNA cloning. A search of the BLAST data base for identification of protein sequence similarities revealed that the sequence of OPA did not manifest any resemblance to fern lectins nor other lectins such as mannose-binding lectins, further implying that OPA was unlikely to be derived from the mannose-binding lectins. Interestingly, amino acid sequence alignment of OPA with two reported true fern lectins, CRA and PAL (PALa and PALb), showed that OPA was about 6%, 4%, and 6% identical to CRA, PALa, and PALb, respectively (Fig. 8). Meanwhile, homologous analysis also showed that the identity between CRA and PAL (PALa and PALb) was 3% and 11%, respectively. As a result, every both of their homology was low. As reported, PAL belonged to Jaclin-related lectins, while CRA was a member of cyanovirin-N (CV-N) homolog family (CVNH) of lectins [52], most members of which showed no significant similarity with known proteins. However, OPA shared a high sequence similarity with syf2 family proteins, which were involved in both cell cycle progression and pre-mRNA splicing. This strange phenomenon might be interpreted as amino acid sequence was not the main factor to determine the function of a protein. The in-depth study of the structure of OPA contributes to more intensive and comprehensive understanding.
In summary, a new lectin had been isolated and its cDNA sequence had been successfully cloned from the root of O. pedunculosum. It was a novel mannan-specific agglutinin with potentially exploitable activities. The OPA showed antifungal activity so the lectin deserved to be a potential agent for plant fungus. Although the specificity of OPA as described here did not suggest any other unique utility of this particular lectin at this time, its distinct character was nevertheless of interest in understanding lectin-binding interactions. Its applications in biology and agriculture, by virtue of their specificities toward mannan and glycoprotein, might also be significant. Future studies will be aimed at the structure and more function of OPA to have a better understanding of its uniqueness. It may provide more promising insights into biotechnological applications to increase fungal resistance of important crops in the future.
References
Suseelan, K. N., Mitra, R., Pandey, R., Sainis, K. B., & Krishna, T. G. (2002). Archives of Biochemistry and Biophysics, 407, 241–247.
Ciopraga, J., Gozia, O., Tudor, R., Brezuica, L., & Doyle, R. (1999). Biochimica et Biophysica Acta, 1428, 424–432.
Rüdiger, H. (1997). In H. J. Gabius & S. Gabiu (Eds.), Glycosciences: status and perpectives, Chap. 23: Structure and function of plant lectins (pp. 415–438). Weinheim: Chapman & Hall.
Rüdiger, H. (1998). Acta Anatomica, 161, 130–152.
Guzmán-Partida, A. M., Robles-Burgueño, M. R., Ortega-Nieblas, M., & Vázquez-Moreno, I. (2004). Biochimie, 86, 335–342.
Sharon, N., & Lis, H. (1972). Science, 177, 949–959.
Liu, B., Bian, H. J., & Bao, J. K. (2010). Cancer Letters, 287, 1–12.
Van Damme, E. J. M., Lannoo, N., & Peumans, W. J. (2008). In J. C. Kader & M. Delseny (Eds.), Advances in botanical research, lant lectins (vol. 48, pp. 107–209). San Diego: Elsevier Science.
Lis, H., & Sharon, N. (1986). Annual Review of Biochemistry, 55, 35–67.
Moreira, R. A., Ainouz, I. L., Oliveira, J. T. A., & Cavada, B. S. (1991). Memórias do Instituto Oswaldo Cruz, 86, 211–218.
Utarabhand, P., & Akkayanont, P. (1995). Phytochemistry, 38, 281–285.
Sharon, N., & Lis, H. (1989). Lectins (1st ed.). London: Chapman & Hall.
Brandley, B. K., Swiedler, S. J., & Robbins, P. W. (1990). Cell, 63, 861–863.
Barral Netto, M., Santos, S. B., Barral, A., Moreira, L. I. M., Santos, C. F., Moreira, R. A., et al. (1992). Immunological Investigations, 21, 297–303.
Konozy, E. H. E., Mulay, R., Faca, V., Ward, R. J., Greene, L. J., Roque-Barriera, M. C., et al. (2002). Biochimie, 84, 1035–1043.
Peumans, W. J., & Van Damme, E. J. M. (1995). Plant Physiology, 109, 347–352.
Liu, Q. H., Wang, H. X., & Ng, T. B. (2004). Peptides, 25, 7–10.
Wang, H., Gao, J., & Ng, T. B. (2000). Biochemical and Biophysical Research Communications, 275, 810–816.
Weis, W. I., & Drickamer, K. (1996). Annual Review of Biochemistry, 65, 441–473.
Dombrovska, O., & Qiu, Y. L. (2004). Molecular Phylogenetics and Evolution, 32, 246–263.
Hauk, W. D., Parks, C. R., & Chase, M. W. (2003). Molecular Phylogenetics and Evolution, 28, 131–151.
Kramer, K. U., & Green, P. S. (1990). In K Kubitzki (Ed.), The families and genera of vascular plants, vol. I: Pteridophytes and gymnosperms (pp. 193–197). Berlin, Heidelberg: Springer-Verlag.
Wu, S. H., Luo, X. D., Ma, Y. B., Hao, X. J., & Wu, D. G. (2002). Acta Pharmacologica Sinica, 37, 33–36.
Laemmli, U. K. (1970). Nature, 227, 680–685.
Andrew, P. (1970). Methods of Biochemical Analysis, 18, 1–6.
Laremore, T. N., Zhang, F., & Linhardt, R. J. (2007). Analytical Chemistry, 79, 1604–1610.
Lam, S. S., Wang, H., & Ng, T. B. (1998). Biochemical and Biophysical Research Communications, 253, 135–142.
Wang, H. X., Ng, T. B., Liu, W. K., Ooi, V. E. C., & Chang, S. T. (1995). International Journal of Peptide and Protein Research, 46, 508–513.
Spande, T. F., & Witkop, B. (1967). Methods in Enzymology, 11, 498–506.
Komath, S. S., Nadimpalli, S. K., & Swamy, M. J. (1998). Biochemisty and Molecular Biology International, 44, 107–116.
Laszlo, P., & Smith, E. L. (1975). The Journal of Biological Chemistry, 25, 557–564.
Gao, S., An, J., Wu, C. F., Gu, Y., Chen, F., Yu, Y., et al. (2005). Acta Biochimica et Biophyica Sinica, 37, 47–54.
Ye, X. Y., Ng, T. B., Tsang, P. W. K., & Wang, J. (2001). Journal of Protein Chemistry, 20, 367–375.
Lam, S. K., & Ng, T. B. (2001). Archives of Biochemistry and Biophysics, 393, 271–280.
Mellor, R. B., Gadd, G. M., Rowell, P., & Stewart, W. D. P. (1981). Biochemical and Biophysical Research Communications, 99, 1348–1353.
McCowen, S. M., MacArthur, L., & Gates, J. E. (1987). Current Microbiology, 14, 329–333.
Tateno, H., Winter, H. C., Petryniak, J., & Goldstein, I. J. (2003). The Journal of Biological Chemistry, 278, 10891–10899.
Yu, P., Liu, Y. R., & Zheng, Y. (2004). Chinese Journal of Applied and Environmental Biology, 10, 740–744.
Yu, P., Liu, Y. R., & Zheng, Y. (2003). Journal of Plant Resources and Environment, 12, 6–9.
Liu, Y. R., Yu, P., & Zheng, Y. (2003). Journal of Plant Physiology, 39, 647–650.
Yu, P., Zheng, Y., & Liu, Y. R. (2004). Journal of Tropical and Subtropical Botany, 12, 57–62.
Percudani, R., Montanini, B., & Ottonello, S. (2005). Proteins: Structure, Function, and Bioinformatics, 60, 670–678.
Banerjee, N., Sengupta, S., Roy, A., Ghosh, P., Das, K., & Das, S. (2011). PloS One, 6, e18593.
Gaidamashvili, M., Ohizumi, Y., Iijima, S., Takayama, T., Ogawa, T., & Muramoto, K. (2004). The Journal of Biological Chemistry, 279, 26028–26035.
Mo, H., Rice, K. G., Evers, D. L., Winter, H. C., Peumans, W. J., Damme, E. J. M. V., et al. (1999). The Journal of Biological Chemistry, 274, 33300–33305.
Yoshima, H., Matsumoto, A., Mizuochi, T., Kawasaki, T., & Kobata, A. (1981). The Journal of Biological Chemistry, 256, 8476–8484.
Liu, C., Zhao, X., Xu, X. C., Li, L. R., Liu, Y. H., Zhong, S. D., et al. (2008). International Journal of Biological Macromolecules, 42, 138–144.
Devyani, N., & Mala, R. (1998). Biochemical and Biophysical Research Communications, 249, 207–212.
Li, Y. R., Liu, Q. H., Wang, H. X., & Ng, T. B. (2008). Biochimica et Biophysica Acta, 1780, 51–57.
Bowman, S. M., & Free, S. J. (2006). Bioassays, 28, 799–808.
Kim, Y., Nandakumar, M. P., & Marten, M. R. (2007). Trends in Biotechnology, 25, 395–400.
Koharudin, L., Viscomi, A., Jee, J., Ottonello, S., & Gronenborn, A. (2008). Structure, 16, 570–584.
Acknowledgements
This work was supported by grants from the National Key Science and Technology Projects Fund (2009ZX8009-072B), the National Natural Science Foundation of China (General Programs: Nos. 30670469 and 30970643). We gratefully acknowledge the contribution and enthusiasm of our coworkers and collaborators in our laboratories in the course of this investigation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xue-mei He and Na Ji contributed equally to this work
Rights and permissions
About this article
Cite this article
He, Xm., Ji, N., Xiang, Xc. et al. Purification, Characterization, and Molecular Cloning of a Novel Antifungal Lectin From the Roots of Ophioglossum pedunculosum . Appl Biochem Biotechnol 165, 1458–1472 (2011). https://doi.org/10.1007/s12010-011-9367-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9367-z