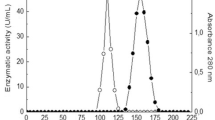
Abstract
Significant differences on structure, stability, and catalytic properties of tannase were found when this enzyme was produced under solid-state and submerged fermentations (SSF and SmF) by Aspergillus niger. The specific activity was 5.5 times higher on SSF than in SmF. Significant differences in isoelectric points of tannases were found. The pH optima for both types of enzyme was found at 6 and the pH stability of SSF and SmF tannase were at 6 and 5–8, respectively. The optimal temperature range was from 50 to 60 °C for SmF tannase and 60 °C for SSF tannase, and both enzyme types showed tolerance to high temperatures (60–70 °C). The SSF tannase showed a major specificity for methyl gallate substrate while SmF tannase for tannic acid. All metal ions tested, had an activity inhibition from 30–46% on SSF tannase. SDS-PAGE analysis as well as gel localization studies of both SSF and SmF purified tannases showed a single band with a molecular weight of 102 and 105 kDa, respectively. Different levels of glycosylation were found among SSF and SmF purified tannases. This is the first report about structural differences among tannase produced under SSF and SmF and this study provides basis for explanation of the stability and catalytic differences observed previously for this two tannase types.






Similar content being viewed by others
References
Adachi, O., Watanabe, M., & Yamada, H. (1968). Studies on fungal tannase. Part II. Physicochemical properties of tannase of Aspergillus flavus. J Agric Food Chem, 32, 1079–1085.
Aguilar, C. N., Augur, C., Favela-Torres, E., & Viniegra-González, G. (2001). Production of tannase by Aspergillus niger Aa-20 in submerged and solid-state fermentation: Influence of glucose and tannic acid. J Ind Microbiol Biotechnol, 26, 296–302.
Aguilar, C. N., Augur, C., Favela-Torres, E., & Viniegra-González, G. (2001). Induction and repression patterns of fungal tannase in solid-state and submerged cultures. Process Biochem, 36, 565–570.
Aguilar, C. N., & Gutiérrez-Sánchez, G. (2001). Review: Sources, properties, applications and potential uses of tannin acyl hydrolase. Food Sci Technol Int, 7, 373–382.
Aguilar, C. N., Gutiérrez-Sánchez, G., Prado-Barragán, L. A., Rodríguez-Herrera, R., Martínez-Hernandez, J. L., & Contreras-Esquivel, J. C. (2010). Perspectives of solid state fermentation for production of food enzymes. Am J Biochem Biotechnol, 4, 354–366.
Aguilar, C. N., Rodríguez, R., Gutiérrez-Sánchez, G., Augur, C., Favela-Torres, E., Prado-Barragan, L. A., et al. (2007). Microbial tannases: Advances and perspectives. Appl Microbiol Biotechnol, 76, 47–59.
Albertse EH (2002) Cloning, expression and characterization of tannase from Aspergillus species. Ms Thesis, University of the Free State, Bloemfontein, South Africa
Aoki, K., Shinke, R., & Nishira, H. (1976). Chemical composition and molecular weight of yeast tannase. Agric Biol Chem, 40, 297–302.
Ayed, L., & Hamdi, M. (2002). Culture conditions of tannase production by Lactobacillus plantarum. Biotechnol Lett, 24, 1763–1765.
Barthomeuf, C., Regerat, F., & Pourrat, H. (1994). Production, purification and characterization of a tannase from Aspergillus niger LCF 8. J Ferment Bioeng, 77, 320–323.
Beena, P. S., Soorej, M. B., Elyas, K. K., Bhat Sarita, G., & Chandrasekaran, M. (2010). Acidophilic tannase from marine Aspergillus awamori BTMFW032. J Microbiol Biotechnol, 20, 1403–1414.
Belur, P. D., Gopal, M., Nirmala, K. R., & Basavaraj, N. (2010). Production of novel cell-associated tannase from newly isolated Serratia ficaria DTC. J Microbiol Biotechnol, 20, 732–736.
Benoit, I., Asther, M., Sulzenbacher, G., Record, E., Marmuse, L., Parsiegla, G., et al. (2006). Respective importance of protein folding and glycosylation in the thermal stability of recombinant feruloyl esterase A. FEBS Lett, 580, 5815–5821.
Beverini, M., & Metche, M. (1990). Identification, purification, physiochemical properties of tannase from Aspergillus oryzae. Sci Aliments, 10, 807–816.
Bhardwaj, R., Singh, B., & Bhat, T. K. (2003). Purification and characterization of tannin acyl hydrolase from Aspergillus niger MTCC 2425. J Basic Microbiol, 43, 449–461.
Bhat, T. K., Singh, B., & Sharma, O. P. (1998). Microbial degradation of tannins—A current perspective. Biodegradation, 9, 343–357.
Blum, H., Beier, H., & Gross, H. J. (1987). Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis, 8, 93–99.
Böer, E., Bode, R., Mock, H. P., Piontek, M., & Kunze, G. (2009). Atan1p—An extracellular tannase from the dimorphic yeast Arxula adeninivorans: Molecular cloning of the ATAN1 gene and characterization of the recombinant enzyme. Yeast, 26, 323–337.
Bradoo, S., Gupta, R., & Saxena, R. K. (1997). Parametric optimization and biochemical regulation of extracellular tannase from Aspergillus japonicus. Process Biochem, 32, 135–139.
Costa, A. M., Ribeiro, W. X., Kato, E., Monteiro, A. R. G., & Peralta, R. M. (2008). Production of tannase by Aspergillus tamarii in submerged cultures. Braz Arch Biol Technol, 51, 399–404.
Curiel, J. A., Rodríguez, H., Acebrón, I., Mancheño, J. M., De Blanca Rivas, L., & Muñoz, R. (2009). Production and physicochemical properties of recombinant Lactobacillus plantarum tannase. J Agric Food Chem, 57, 6224–6230.
Das Mohapatra, P. K., Maity, C., Rao, R. S., Pati, B. R., & Mondal, K. C. (2009). Tannase production by Bacillus licheniformis KBR6: Optimization of submerged culture conditions by Taguchi DOE methodology. Food Res Int, 42, 430–435.
Enemuor, S. C., & Odibo, F. J. C. (2009). Culture conditions for the production of a tannase of Aspergillus tamarii IMI388810. Afr J Biotechnol, 8, 2554–2557.
Hata, Y., Ishida, H., Ichikawa, E., Kawato, A., Suginami, K., & Imayasu, S. (1998). Nucleotide sequence of an alternative glucoamylase-encoding gene (glaB) expressed in solid-state culture of Aspergillus oryzae. Gene, 207, 127–134.
Hatamoto, O., Watarai, T., Kikuchi, M., Mizusawa, K., & Sekine, H. (1996). Cloning and sequencing of the gene encoding tannase and a structural study of the tannase subunit from Aspergillus oryzae. Gene, 175, 215–221.
Iwashita, K. (2002). Recent studies of protein secretion by filamentous fungi. J Biosci Bioeng, 94, 530–535.
Kasieczka-Burnecka, M., Kuc, K., Kalinowska, H., Knap, M., & Turkiewicz, M. (2007). Purification and characterization of two cold-adapted extracellular tannin acyl hydrolases from an Antarctic strain Verticillium sp. P9. Appl Microbiol Biotechnol, 77, 77–89.
Kumar, R. A., Gunasekaran, P., & Lakshmanan, M. (1999). Biodegradation of tannic acid by Citrobacter freundii isolated from a tannery effluent. J Basic Microbiol, 39, 161–168.
Lekha, P. K., & Lonsane, B. K. (1994). Comparative titres, location and properties of tannin acyl hydrolase produced by Aspergillus niger PKL 104 in solid-state, liquid surface an submerged fermentations. Process Biochem, 29, 497–503.
Lekha, P. K., & Lonsane, B. K. (1997). In S. Neidleman & A. Laskin (Eds.), Advances in Applied Microbiology vol. 44 (pp. 215–260). San Diego: Academic.
Mahapatra, K., Nanda, R. K., Bag, S. S., Banerjee, R., Pandey, A., & Szakacs, G. (2005). Purification, characterization and some studies on secondary structure of tannase from Aspergillus awamori nakazawa. Process Biochem, 40, 3251–3254.
Manjit, Yadav, A., Aggarwal, N. K., Kumar, K., & Kumar, A. (2008). Tannase production by Aspergillus fumigatus MA under solid-state fermentation. World J Microbiol Biotechnol, 24, 3023–3030.
Mata-Gomez, M. A., Rodríguez, L. V., Ramos, E. L., Renovato, J., Cruz-Hernández, M. A., Rodríguez, R., et al. (2009). A novel tannase from the xerophilic fungus Aspergillus niger GH1. J Microbiol Biotechnol, 19, 987–996.
Mohanasrinivasan, V., Dhrisya, P., Dipinsha, K. P., Unnithan, C. M., Viswanath, K. M., & Devi, C. S. (2009). A comparative study of the lipase yield by solid state and submerged fermentations using fungal species from biopharmaceutical oil waste. Afr J Biotechnol, 8, 73–76.
Mondal, K. C., & Pati, B. R. (2000). Studies on the extracellular tannase from newly isolated Bacillus licheniformis KBR 6. J Basic Microbiol, 40, 223–232.
Natarajan, K., & Rajendran, A. (2009). Effect of fermentation parameters on extra cellular tannase production by Lactobacillus plantarum MTCC 1407. E J Chem, 6, 979–984.
Prasad D, Gupta RK, Naidu RB, Kamini NR, Gowthaman MK (2008) Solid state fermentation of tannase from a new Aspergillus isolate. 49th Annual Conference of Association of Microbiologists of India. New Delhi, India.
Rajakumar, G., & Nandy, S. C. (1983). Isolation, purification, and some properties of Penicillium chrysogenum tannase. Appl Environ Microbiol, 46, 525–527.
Ramírez-Coronel, M. A., Viniegra-González, G., Darvill, A., & Augur, C. (2003). A novel tannase from Aspergillus niger with β-glucosidase activity. Microbiology, 149, 2941–2946.
Rana, N. K., & Bhat, T. K. (2005). Effect of fermentation system on the production and properties of tannase of Aspergillus niger van Tieghem MTCC 2425. J Gen Appl Microbiol, 51, 203–212.
Rodríguez, H., de las Rivas, B., Gómez-Cordovés, C., & Muñoz, R. (2008). Characterization of tannase activity in cell-free extracts of Lactobacillus plantarum CECT 748T. Int J Food Microbiol, 121, 92–98.
Saqib, A. A. N., Hassan, M., Khan, N. F., & Baig, S. (2010). Thermostability of crude endoglucanase from Aspergillus fumigatus grown under solid state fermentation (SSF) and submerged fermentation (SmF). Process Biochem, 45, 641–646.
Sharma, S., Bhat, T. K., & Dawra, R. K. (1999). Isolation, purification and properties of tannase from Aspergillus niger van Tieghem. World J Microbiol Biotechnol, 15, 673–677.
Sharma, S., & Gupta, M. N. (2003). Synthesis of antioxidant propyl gallate using tannase from Aspergillus niger van Teighem in nonaqueous media. Bioorg Med Chem Lett, 13, 395–397.
Strumeyer, D. H., & Malin, M. J. (1970). Resistance of extracellular yeast invertase and other glycoproteins to denaturation by tannins. Biochem J, 118, 899–900.
Taşkın, E., & Stratilová, E. (2008). Polygalacturonases produced under solid state and submerged fermentation conditions by two strains of Aspergillus foetidus. Turk J Biochem, 33, 190–196.
Vaquero, I., Marcobal, A., & Muñoz, R. (2004). Tannase activity by lactic acid bacteria isolated from grape must and wine. Int J Food Microbiol, 96, 199–204.
Viniegra-González, G., Favela-Torres, E., Aguilar, C., Romero-Gomez, S. D. J., Díaz-Godínez, G., & Augur, C. (2003). Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem Eng J, 13, 157–167.
Yamada, K., Iibuchi, S., & Minoda, Y. (1968). Studies on tannin acyl hydrolase of microorganisms. Isolation and identification of producing molds and studies on the conditions of cultivation. Agric Biol Chem, 45, 233–240.
Yu, X. W., & Li, Y. Q. (2006). Kinetics and thermodynamics of synthesis of propyl gallate by mycelium-bound tannase from Aspergillus niger in organic solvent. J Mol Catal B Enzym, 40, 44–50.
Acknowledgments
Jaqueline Renovato and Luis Rodríguez-Durán thank the National Council on Science and Technology (CONACYT), Mexico for the post-graduate scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Renovato, J., Gutiérrez-Sánchez, G., Rodríguez-Durán, L.V. et al. Differential Properties of Aspergillus niger Tannase Produced Under Solid-State and Submerged Fermentations. Appl Biochem Biotechnol 165, 382–395 (2011). https://doi.org/10.1007/s12010-011-9258-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9258-3