Abstract
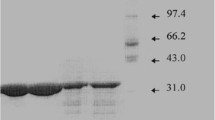
An organic solvent-stable protease from Pseudomonas aeruginosa PT121 was purified in a single step with 55% recovery by hydrophobic interaction chromatography on a Phenyl Sepharose High Performance matrix. The purified protease was homogenous on SDS-PAGE and had an estimated molecular mass of 33 kDa. The optimal pH and temperature conditions for enzyme activity were 8.0 and 60°C, respectively. The enzyme was classified as a metalloprotease based on its strong inhibition by EDTA and 1,10-phenanthroline and exhibited good stability across a broad pH range (6.0–11.0). The protease was quite stable in the presence of various water-miscible organic solvents. This is a unique property of the protease which makes it an ideal choice for application in aqueous-organic phase organic synthesis including peptides synthesis. The synthetic activity of the protease was tested using N-carbobenzoxy-l-asparagine (Z-Asp) and l-phenylalaninamide (Phe-NH2) as substrate in the presence of various water-miscible organic solvents for aspartame precursor synthesis. The highest yield was obtained in the presence of 50% DMSO (91%). The synthesis rate in the presence of DMSO was also much higher than the rates in the other tested organic solvents, and the initial rates of Z-Asp-Phe-NH2 synthesis in mixtures of various water-miscible organic solvents, with the exception of ethanol, correlated with the yields of Z-Asp-Phe-NH2. Furthermore, the PT121 protease was able to use various carboxyl components (Z-AA) and Phe-NH2 as substrates to catalyze the syntheses of the dipeptides, indicating that this protease has a broad specificity for carboxylic acid residue.






Similar content being viewed by others
References
Gupta, R., Beg, Q., & Lorenz, P. (2002). Applied Microbiology and Biotechnology, 59, 15–32. doi:10.1007/s00253-002-0975-y.
Bordusa, F. (2002). Chemical Reviews, 102, 4817–4868. doi:10.1021/cr010164d.
Kumar, D., & Bhalla, T. C. (2005). Applied Microbiology and Biotechnology, 68, 726–736. doi:10.1007/s00253-005-0094-7.
Ogino, H., & Ishikawa, H. (2001). Journal of Bioscience and Bioengineering, 91, 109–116. doi:10.1263/jbb.91.109.
Sardessai, Y. N., & Bhosle, S. (2004). Biotechnology Progress, 20, 655–660. doi:10.1021/bp0200595.
Heipieper, H. J., Neumann, G., Cornelissen, S., & Meinhardt, F. (2007). Applied Microbiology and Biotechnology, 74, 961–973. doi:10.1007/s00253-006-0833-4.
Rahman, R., Mahamad, S., Salleh, A. B., & Basri, M. (2007). Journal of Industrial Microbiology & Biotechnology, 34, 509–517. doi:10.1007/s10295-007-0222-8.
Doddapaneni, K. K., Tatineni, R., Vellanki, R. N., Rachcha, S., Anabrolu, N., Narakuti, V., et al. (2007). Microbiological Research, . doi:10.1016/j.micres.2007.04.005.
Ghorbel, B., Sellami-Kamoun, A., & Nasri, M. (2003). Enzyme and Microbial Technology, 32, 513–518. doi:10.1016/S0141-0229(03)00004-8.
Li, S., He, B. F., Bai, Z. Z., & Ouyang, P. K. (2008). Journal of Molecular Catalysis B, Enzymatic, 56, 85–88. doi:10.1016/j.molcatb.2008.08.001.
Rahman, R., Geok, L. P., Basri, M., & Salleh, A. B. (2006). Enzyme and Microbial Technology, 39, 1484–1491. doi:10.1016/j.enzmictec.2006.03.038.
Ogino, H., Watanabe, F., Yamada, M., Nakagawa, S., Hirose, T., Noguchi, A., et al. (1999). Journal of Bioscience and Bioengineering, 87, 61–68. doi:10.1016/S1389-1723(99)80009-7.
Gupta, A., & Khare, S. K. (2006). Bioresource Technology, 97, 1788–1793. doi:10.1016/j.biortech.2005.09.006.
Ogino, H., Yamada, M., Watanabe, F., Ichinose, H., Yasuda, M., & Ishikawa, H. (1999). Journal of Bioscience and Bioengineering, 88, 513–518. doi:10.1016/S1389-1723(00)87668-9.
Sareen, R., Bornscheuer, U. T., & Mishra, P. (2004). Journal of Molecular Catalysis B, Enzymatic, 32, 1–5. doi:10.1016/j.molcatb.2004.09.006.
Sareen, R., & Mishra, P. (2008). Applied Microbiology and Biotechnology, 79, 399–405. doi:10.1007/s00253-008-1429-y.
Tang, X. Y., Pan, Y., Li, S., & He, B. F. (2008). Bioresource Technology, 99, 7388–7392. doi:10.1016/j.biortech.2008.01.030.
Shimogaki, H., Takeuchi, K., Nishino, T., Ohdera, M., Kudo, T., Ohba, K., et al. (1991). Agricultural and Biological Chemistry, 55, 2251–2258.
Bradford, M. M. (1976). Analytical Biochemistry, 72, 248–254. doi:10.1016/0003-2697(76)90527-3.
Laemmli, U. K. (1970). Nature, 227, 680. doi:10.1038/227680a0.
Gupta, A., Roy, I., Patel, R. K., Singh, S. P., Khare, S. K., & Gupta, M. N. (2005). Journal of Chromatography A, 1075, 103–108. doi:10.1016/j.chroma.2005.03.127.
Wang, S. L., & Yeh, P. Y. (2006). Process Biochemistry, 41, 1545–1552. doi:10.1016/j.procbio.2006.02.018.
Gupta, A., Roy, I., Khare, S. K., & Gupta, M. N. (2005). Journal of Chromatography A, 1069, 155–161. doi:10.1016/j.chroma.2005.01.080.
Nicas, T. I., & Iglewski, B. H. (1985). Canadian Journal of Microbiology, 31, 387–392.
Umeki, S. (1989). Journal of Medical Microbiology, 28, 109–112.
Schokker, E. P., & van Boekel, M. A. J. S. (1997). International Dairy Journal, 7, 165–171. doi:10.1016/S0958-6946(97)00008-3.
Wang, S. L., Kao, T. Y., Wang, C. L., Yen, Y. H., Chern, M. K., & Chen, Y. H. (2006). Enzyme and Microbial Technology, 39, 724–731. doi:10.1016/j.enzmictec.2005.12.007.
Sierecka, J. K. (1998). The International Journal of Biochemistry & Cell Biology, 30, 579–595. doi:10.1016/S1357-2725(98)00007-7.
Maurer, K. H. (2004). Current Opinion in Biotechnology, 15, 330–334. doi:10.1016/j.copbio.2004.06.005.
Zaks, A., & Klibanov, A. M. (1988). The Journal of Biological Chemistry, 263, 8017–8021.
Ogino, H., Nakagawa, S., Shinya, K., Muto, T., Fujimura, N., Yasuda, M., et al. (2000). Journal of Bioscience and Bioengineering, 89, 451–457. doi:10.1016/S1389-1723(00)89095-7.
Karbalaei-Heidari, H. R., Ziaee, A. A., & Amoozegar, M. A. (2007). Extremophiles, 11, 237–243. doi:10.1007/s00792-006-0031-4.
Klibanov, A. M. (2001). Nature, 409, 241–246. doi:10.1038/35051719.
Gupta, A., & Khare, S. K. (2007). Enzyme and Microbial Technology, 42, 11–16. doi:10.1016/j.enzmictec.2007.07.019.
Zhou, Y. Y., Yang, T., Wang, N., Xu, L., Huang, Y. B., Wu, X. X., et al. (2003). Enzyme and Microbial Technology, 33, 55–61. doi:10.1016/S0141-0229(03)00095-4.
Murakami, Y., Yoshida, T., Hayashi, S., & Hirata, A. (2002). Biotechnology and Bioengineering, 69, 57–65. doi:10.1002/(SICI)1097-0290(20000705)69:1<57::AID-BIT7>3.0.CO;2-J.
Voyushina, T. L., Potetinova, J. V., Milgotina, E. I., & Stepanov, V. M. (1999). Bioorganic & Medicinal Chemistry, 7, 2953–2959. doi:10.1016/S0968-0896(99)00237-0.
Toledano, S., Williams, R. J., Jayawarna, V., & Ulijn, R. V. (2006). Journal of the American Chemical Society, 128, 1070–1071. doi:10.1021/ja056549l.
Acknowledgements
This study was supported by the National Basic Research Program of China (No. 2004CB719600) and the National High Technology Research and Development Program of China (No. 2006AA02Z202).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, XY., Wu, B., Ying, HJ. et al. Biochemical Properties and Potential Applications of a Solvent-Stable Protease from the High-Yield Protease Producer Pseudomonas aeruginosa PT121. Appl Biochem Biotechnol 160, 1017–1031 (2010). https://doi.org/10.1007/s12010-009-8665-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8665-1