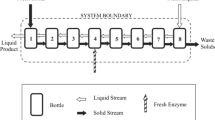
Abstract
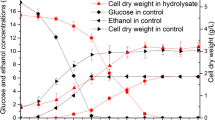
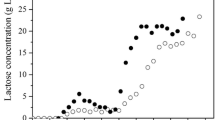
An operable batch model of simultaneous saccharification and fermentation (SSF) for ethanol production from cellulose has been developed. The model includes four ordinary differential equations that describe the changes of cellobiose, glucose, yeast, and ethanol concentrations with respect to time. These equations were used to simulate the experimental data of the four main components in the SSF process of ethanol production from microcrystalline cellulose (Avicel PH101). The model parameters at 95% confidence intervals were determined by a MATLAB program based on the batch experimental data of the SSF. Both experimental data and model simulations showed that the cell growth was the rate-controlling step at the initial period in a series of reactions of cellulose to ethanol, and later, the conversion of cellulose to cellobiose controlled the process. The batch model was extended to the continuous and fed-batch operating models. For the continuous operation in the SSF, the ethanol productivities increased with increasing dilution rate, until a maximum value was attained, and rapidly decreased as the dilution rate approached the washout point. The model also predicted a relatively high ethanol mass for the fed-batch operation than the batch operation.





Similar content being viewed by others
Abbreviations
- B :
-
Cellobiose concentration (g/l)
- B 1 :
-
Convertible cellobiose concentration from the reaction r 1 (g/l)
- C :
-
Cellulose concentration (g/l)
- C 0 :
-
Initial cellulose concentration (g/l)
- C 1 :
-
Cellulose concentration in the feed flow (g/l)
- D :
-
Dilution rate (=F/V) (h−1)
- e :
-
Total enzyme concentration (g/l)
- e e :
-
Endo-β-1,4-glucanase and exo-β-1,4-cellobiohydrolase concentration (g/l)
- \( e_{\text{e}} C_{\text{in}}^{ * } \) :
-
Ineffective complex formed by the enzymes and substrate (g/l)
- G :
-
Glucose concentration (g/l)
- F :
-
Feed rate (l/h)
- f :
-
Proportionality constant (dimensionless)
- K 1B :
-
Inhibitory constant of cellobiose to the endo-β-1,4-glucanase and exo-β-1,4-cellobiohydrolase (g/l)
- K 1G :
-
Inhibitory constant of glucose to the endo-β-1,4-glucanase and exo-β-1,4-cellobiohydrolase (g/l)
- K 2G :
-
Inhibitory constant of glucose to the glycosidase (g/l)
- K G :
-
Glucose saturation constant for the microbial growth (g/l)
- k 1 :
-
Specific rate constant of cellulose hydrolysis to cellobiose (l/(g h))
- k ’1 :
-
Variable associated with enzyme deactivation (h−1)
- k 2 :
-
Specific rate constant of cellobiose hydrolysis to glucose (h−1)
- k 3 :
-
Product formation coefficient associated with cell growth (=k ′3 Y G/E) (dimensionless)
- k 4 :
-
Constant (=k ′4 f) (l/(g h))
- k ’4 :
-
Specific rate constant of enzyme deactivation (l/(g h))
- m :
-
Maintenance coefficient for endogenous metabolism of the microorganisms (h−1)
- r 1 :
-
Reaction rate from cellulose to cellobiose (g/(l h))
- r 2 :
-
Reaction rate from cellobiose to glucose (g/(l h))
- r E :
-
Reaction rate of ethanol formation (g/(l h))
- r G :
-
Reaction rate of glucose consumption (g/(l h))
- r X :
-
Reaction rate of cell formation (g/(l h))
- t :
-
Residence time (h)
- V :
-
Liquid volume in reactor (l)
- X :
-
Cell concentration (g/l)
- X 0 :
-
Initial cell concentration (g/l)
- Y G/E :
-
Conversion factor of ethanol from glucose (g/g)
- Y th :
-
Theoretical ethanol yield
- Y X/G :
-
Yield coefficient of cell mass on the glucose (g/g)
- Y X/E :
-
Yield coefficient of cell from ethanol (g/g)
- μ :
-
Specific cell growth rate constant (h−1)
- μ m :
-
Maximum specific cell growth rate constant (h−1)
- ’ :
-
Overall masses in the culture (g/l)
References
Philippidis, G. P., Spindler, D. D., & Wyman, C. E. (1992). Applied Biochemistry and Biotechnology, 34–35, 543–556. doi:10.1007/BF02920577.
Philippidis, G. P., Smith, T. K., & Wyman, C. E. (1993). Biotechnology and Bioengineering, 41(9), 846–853. doi:10.1002/bit.260410903.
South, C. R., Hogsett, D. A. L., & Lynd, L. R. (1995). Enzyme and Microbial Technology, 17(9), 797–803. doi:10.1016/0141-0229(94)00016-K.
Philippidis, G. P., & Hatzis, C. (1997). Biotechnology Progress, 13(3), 222–231. doi:10.1021/bp970017u.
Shin, D., Yoo, A., Kim, S. W., & Yang, D. R. (2006). Journal of Microbiology and Biotechnology, 16(9), 1355–1361.
Moon, H., Kim, J. S., Oh, K. K., Kim, S. W., & Hong, S. I. (2001). Journal of Microbiology and Biotechnology, 4, 598–606.
Pettersson, P. O., Eklund, R., & Zacchi, G. (2002). Applied Biochemistry and Biotechnology, 98, 733–746. doi:10.1385/ABAB:98-100:1-9:733.
Rudolf, A., Alkasrawi, M., Zacchi, G., & Liden, G. (2005). Enzyme and Microbial Technology, 37(2), 195–204. doi:10.1016/j.enzmictec.2005.02.013.
Zhu, S. D., Wu, Y. X., Zhao, Y. F., Tu, S. Y., & Xue, Y. P. (2006). Chemical Engineering Communications, 193(5), 639–648. doi:10.1080/00986440500351966.
Lehninger, A. L., Nelson, D. L., & Cox, M. M. (1993). Principles of biochemistry (2nd ed., p. 229). New York: Worth.
Shen, J. C., & Agblevor, F. A. (2008). Biochemical Engineering Journal, 41, 241–250. doi:10.1016/j.bej.2008.05.001.
Shuler, M. L., & Kargi, F. (2002). Bioprocess engineering (2nd ed., p. 217). New York: Prentice Hall.
Ghose, T. K. (1987). Pure and Applied Chemistry, 59(2), 257–268. doi:10.1351/pac198759020257.
Levenspiel, O. (1999). Chemical reaction engineering (3rd ed., p. 171). New York: Wiley.
Kosaric, N., & Vardar-Sukan, F. (2001). Potential source of energy and chemical products. In M. Roehr (Ed.), Biotechnology of ethanol: Classical and future applications (p. 180). New York: Wiley.
Ohgren, K., Bura, R., Lesnicki, G., Saddler, J., & Zacchi, G. (2007). Process Biochemistry, 42(5), 834–839. doi:10.1016/j.procbio.2007.02.003.
Glazer, A. N., & Nikaido, H. (1995). Microbial biotechnology (p. 366). New York: W. H. Freedom and Company.
Blanch, H. W., & Clark, D. S. (1996). Biochemical engineering (p. 290). New York, NY: Marcel Dekker.
Stanburg, P. F., Whitaker, A., & Hall, S. J. (1995). Principles of fermentation technology (2nd ed., p. 23). Elsevier: Amsterdam.
Pirt, S. J. (1979). Fed-batch culture of microbes. Annals of the New York Academy of Sciences, 326, 119–125. doi:10.1111/j.1749-6632.1979.tb14156.x.
Acknowledgments
The authors acknowledge the National Science Foundation (NSF) under contract No. 0420577 and Xethanol Inc. for providing financial support for this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, J., Agblevor, F.A. The Operable Modeling of Simultaneous Saccharification and Fermentation of Ethanol Production from Cellulose. Appl Biochem Biotechnol 160, 665–681 (2010). https://doi.org/10.1007/s12010-009-8650-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8650-8