Abstract
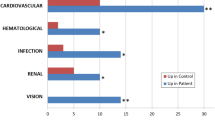
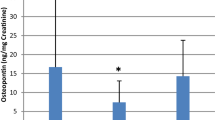
Renal calculi disease or known as kidney stone disease is the most common urological disorder in both men and women, although it is more prevalent in men. The lifetime chance for an individual to develop renal calculi is ~10% whereas the risk of recurrence in a 10-year period is 74%. Therefore, a diagnostic tool for screening or detecting renal calculi is greatly needed. In this study, we analyze urinary protein profiles from patients with renal calculi for the first time (RC), healthy subjects (HS), and patients with recurrent renal calculi (RRC) to identify a biomarker for detecting the disease. Urinary proteins were isolated by salt precipitation and the proteins resolved by SDS-PAGE. Target proteins were analyzed with LC/MS/MS. Thirty-two proteins were identified from healthy subjects and patients. Uromodulin was the most abundant urinary protein in HS but was a very faint band if detected at all from those that formed renal calculi for the first time (p < 0.05). Yet the excreted levels of urinary uromodulin in RRC were similar to those of the HS suggesting that uromodulin is a reliable biomarker for only RC. In addition, a few immunoglobulins that were commonly found in the urine of both RC and RRC, which include Ig alpha heavy chain 1, Ig gamma-2 c region, Ig gamma-3 heavy chain disease protein, Ig heavy chain variable region, Ig heavy constant region gamma 4, and Ig heavy chain. Ig heavy chain Fab frag and antibody a5b7 chain B may serve as potential biomarkers for renal calculi disease.




Similar content being viewed by others
References
Barbas, C., García, A., Saavedra, L., & Muros, M. (2002). Urinary analysis of nephrolithiasis markers. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 781(1–2), 433–455. doi:10.1016/S1570-0232(02)00557-3.
Hess, B., & Kok, D. J. (1996). Nucleation, growth, and aggregation of crystals. In F. L. Coe, M. J. Favus, C. Y. Paak, J. H. Parks, & G. M. Preminger (Eds.), Kidney stones. Medical and surgical management. Philadelphia, PA, USA: Lippincott-Raven.
Bihl, G., & Meyers, A. (2001). Recurrent renal stone disease—advances in pathogenesis and clinical management. Lancet, 358, 651–656. doi:10.1016/S0140-6736(01)05782-8.
Anne, L. T., & Gill, R. (1999). Isoelectric focusing of native urinary uromodulin (Tamm–Horsfall protein) shows no physicochemical differences between stone formers and non-stones formers. Urological Research, 27, 250–254. doi:10.1007/s002400050118.
Lafitte, D., Dussol, B., Andersen, S., Vazi, A., Dupuy, P., Jensen, L. N., Berland, Y., & Verdier, J. M. (2002). Optimized preparation of urine sample from two-dimensional electrophoresis and initial application to patient samples. Clinical Biochemistry, 35, 581–589. doi:10.1016/S0009-9120(02)00362-4.
Tiselius, H. G. (Ed.).(1996) The patient with renal stone disease. Dallas: Millet.
Trewick, A. I., & Rumsby, G. (1999). Isoelectric focusing of native urinary uromodulin (Tamm–Horsfall protein) shows no physicochemical differences between stone formers and non-stone formers. Urological Research, 27, 250–254. doi:10.1007/s002400050118.
Pieper, R., Gatin, C. L., McGrath, A. M., Makusky, A. J., Mondal, M., Seonarain, M., Field, E., Schatz, C. R., Estock, A. M., Ahmed, N., Anderson, N. G., & Steiner, S. (2004). Characterization of the human urinary proteome: a method for high-resolution display of urinary proteins on two-dimensional electrophoresis gels with a yield of nearly 1,400 distinct protein spots. Proteomics, 4, 1159–1174. doi:10.1002/pmic.200300661.
Smith, G., Barratt, D., Rowlinson, R., Nickson, J., & Tonge, R. (2005). Development of a high-throughput method for preparing human urine for two-dimensional electrophoresis. Proteomics, 5, 2315–2318. doi:10.1002/pmic.200401267.
Kshirsagar, B., & Wiggins, R. C. (1986). A map of urine proteins based on one-dimensional SDS-polyacrylamide gel electrophoresis and Western blotting using one microliter of unconcentrated urine. Clinica Chimica Acta, 15, 13–22. doi:10.1016/0009-8981(86)90111-7.
Graves, P. R., & Haystead, T. A. J. (2002). Molecular biologist’s guide to proteomics. Microbiology and Molecular Biology Reviews, 66, 39–63. doi:10.1128/MMBR.66.1.39-63.2002.
Doonan, S. (1996). Protein purification protocols. In S. Doonan (Ed.), Bulk purification by fractional precipitation (pp. 135–150). Totawa, New Jersey: Methods in molecular biology.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the folin phenol reagent. The Journal of Biological Chemistry, 193, 265–275.
Towbin, H., Staehelin, T., & Gordon, J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proceedings of the National Academy of Sciences of the United States of America, 76, 4350–4354. doi:10.1073/pnas.76.9.4350.
Gam, L.-H., Leow, C.-H., Man, C.-N., Gooi, B.-H., & Manjit, S. (2006). Analysis of differentially expressed proteins in cancerous and normal colonic tissues. World Journal of Gastroenterology, 12(31), 4973–4983.
Baldwin, M. A. (2004). Protein identification by mass spectrometry. Molecular & Cellular Proteomics, 3, 1–9. doi:10.1074/mcp.R300012-MCP200.
Sikri, K. L., Foster, C. L., MacHugh, N., & Marshall, R. D. (1981). Localisation of Tamm-Horsfall glycoprotein in the human kidney using immuno-fluorescence and immuno-electron microscopical techniques. Journal of Anatomy, 132, 597.
Hess, B., Nakagawa, Y., & Coe, F. (1989). Inhibition of calcium oxalate monohydrate crystal aggregation by urine proteins. The American Journal of Physiology, 257, F99–F106.
Stevenson, F. K., & Kent, P. W. (1970). Subunits of Tamm–Horsfall glycoprotein. The Biochemical Journal, 116, 791–791.
Sophasan, S., Chatasingh, S., Thanaphaichitr, P., & Dhanamitta, S. (1980). Tamm–Horsfall mucoprotein in urine of potential bladder stone formers. The Journal of Urology, 124, 522–524.
Hess, B., Nakagawa, Y., Perks, J. H., & Coe, F. L. (1991). Molecular abnormality of Tamm–Horsfall glycoprotein in calcium oxalate nephrolithiasis. The American Journal of Physiology, 260, F569–F578.
Bichler, K., Mittermuller, B., Strohmaier, W. L., Feil, G., & Eipper, E. (1999). Excretion of Tamm–Horsfall protein in patients with uric acid stones. Urologia Internationalis, 62, 87–92. doi:10.1159/000030364.
Pourmand, D., Nasseh, H., Sarrafnejad, A., Mojtahedi, , Mehrsai, A., Hamidi-Alamdari, D., & Nourijelyani, K. (2006). Comparison of urinary protein in calcium stone formers and healthy individuals: a case-control study. Urologia Internationalis, 76, 163–168. doi:10.1159/000090882.
Chen, W.-C., Lin, H.-S., Chen, H.-Y., Shih, C.-H., & Li, C.-W. (2001). Effects of Tamm–Horsfall protein and albumin on calcium oxalate crystallization and importance of sialic acids. Molecular Urology, 5, 1–5. doi:10.1089/109153601750124186.
Dussol, B., Geider, S., Lilova, A., Leonetti, F., Dupuy, P., Daudon, M., Berland, Y., Dagorn, J. C., & Verdier, J. M. (1995). Analysis of the soluble organic matrix of five morphologically different kidney stone. Evidence for a specific role of albumin in the constitution of the stone protein matrix. Urological Research, 23, 45–51. doi:10.1007/BF00298850.
Grover, P. K., & Ryall, R. L. (1999). Inhibition of calcium oxalate crystal growth and aggregation by prothrombin and its fragments in vitro: relationship between protein structure and inhibitory activity. European Journal of Biochemistry, 263, 50–56. doi:10.1046/j.1432-1327.1999.00448.x.
Fraij, B. M. (1989). Separation and identification of urinary protein and stone-matrix proteins by mini-slab sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Clinical Chemistry, 35, 658–662.
Umekawa, T., Kohri, K., Amasaki, N., Yamate, T., Yoshida, K., Yamamoto, K., Suzuki, Y., Sinohara, H., & Kurita, T. (1993). Sequencing of a urinary stone protein, identical to alpha-one antitrypsin, which lacks 22 amino acids. Biochemical and Biophysical Research Communications, 193, 1049–1053. doi:10.1006/bbrc.1993.1731.
Tiselius, H.-G., Ackermann, D., Hess, B., & Boevé, E. (2002). Stone disease: diagnosis and medical management. European Urology, 41, 1–11. doi:10.1016/S0302-2838(02)00045-3.
Bakoush, O., Grubb, A., Rippet, B., & Tencer, J. (2001). Urine excretion of protein HC in proteinuric glomerular diseases correlates to urine IgG but not to albuminuria. Kidney International, 60, 1904–1909. doi:10.1046/j.1523-1755.2001.00018.x.
Bazzi, C. (2003). Composition of proteinuria in primary glomerulonephritides: association with tubolo-interstitial damage, outcome and response to therapy. Giornale Italiano di Nefrologia, 20(4), 346–355.
Acknowledgments
We thank the staff and patients of the urology clinic, Penang General Hospital, Lam Wah Ee hospital and the students and staff of the School of Pharmaceutical Sciences, USM for providing the urine specimens for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wai-Hoe, L., Wing-Seng, L., Ismail, Z. et al. Proteomics and Detection of Uromodulin in First-time Renal Calculi Patients and Recurrent Renal Calculi Patients. Appl Biochem Biotechnol 159, 221–232 (2009). https://doi.org/10.1007/s12010-008-8503-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8503-x