Abstract
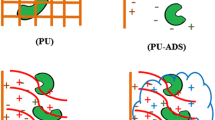
In situ affinity foam fractionation is a potential powerful tool for continuous, selective removal of products from bioprocesses. When evaluating its applicability to cellulase production by Trichoderma reesei fermentation, we encountered the difficulty of significant removal of fungal mycelia along with the cellulase. To solve this problem, cell immobilization using cut pieces of hydrophilic polyurethane (PU) foam was evaluated. Five commercial PU foams with different pore sizes and porosities were tested. Two were found to support good cell growth, cellulase production, and cell loading (about 0.6 g dry cells per g PU). The PU-immobilized mycelia were successfully retained in the foaming process.













Similar content being viewed by others
References
Enari, T. M., & Niku-Paavola, M. L. (1987). Enzymic hydrolysis of cellulose: Is the current theory of the mechanisms of hydrolysis valid? Critical Reviews in Biotechnology, 5(1), 67–87. doi:10.3109/07388558709044153.
Persson, I., Tjerneld, F., & Hahn-Haegerdal, B. (1991). Fungal cellulolytic enzyme production: A review. Process Biochemistry (Oxford, United Kingdom), 26(2), 65–74.
Zhang, Q., Lo, C.-M., & Ju, L.-K. (2006). Affinity foam fractionation of Trichoderma cellulase. Applied Biochemistry and Biotechnology, 129–132, 1051–1065.
Zhang, Q., Lo, C.-M., & Ju, L.-K. (2006). Factors affecting foaming behavior in cellulase fermentation by Trichoderma reesei Rut C-30. Bioresource Technology, 98(4), 753–760. doi:10.1016/j.biortech.2006.04.006.
Ju, L.-K., & Zhang, Q. (2006). Affinity foam fractionation for collection and purification of materials. PCT Int. Appl. Wo (The University of Akron, USA).
Husband, D. L., Masliyah, J. H., & Gray, M. R. (1994). Cell and surfactant separation by column flotation. Canadian Journal of Chemical Engineering, 72(5), 840–847.
Zhang, Q. (2007). Collection of Trichoderma reesei cellulase by foaming. PhD thesis. Chemical and Biomolecular Engineering, The University of Akron, Akron.
Mukataka, S., Kobayashi, N., Sato, S., & Takahashi, J. (1988). Variation in cellulase-constituting components from Trichoderma reesei with agitation intensity. Biotechnology and Bioengineering, 32(6), 760–763. doi:10.1002/bit.260320606.
Reese, E. T., & Ryu, D. Y. (1980). Shear inactivation of cellulase of Trichoderma reesei. Enzyme and Microbial Technology, 2(3), 239–240. doi:10.1016/0141-0229(80)90054-X.
Vorlop, K. D., Muscat, A., & Beyersdorf, J. (1992). Entrapment of microbial cells within polyurethane hydrogel beads with the advantage of low toxicity. Biotechnology Techniques, 6(6), 483–488. doi:10.1007/BF02447818.
Sanroman, A., Pintado, J., & Lema, J. M. (1994). A comparison of two techniques (adsorption and entrapment) for the immobilization of Aspergillus niger in polyurethane foam. Biotechnology Techniques, 8(6), 389–394. doi:10.1007/BF00154309.
Haapala, R., Linko, S., Parkkinen, E., & Suominen, P. (1994). Production of endo-1,4-b-glucanase and xylanase by Trichoderma reesei immobilized on polyurethane foam. Biotechnology Techniques, 8(6), 401–406. doi:10.1007/BF00154311.
Haapala, R., Parkkinen, E., Suominen, P., & Linko, S. (1995). Production of extracellular enzymes by immobilized Trichoderma reesei in shake flask cultures. Applied Microbiology and Biotechnology, 43(5), 815–821. doi:10.1007/BF02431913.
Targonski, Z., & Pielecki, J. (1995). Continuous semi-solid cultivation for the production of cellulase by Trichoderma reesei mutants using a polyurethane foam carrier and a liquid medium. Acta Biotechnologica, 15(3), 289–296. doi:10.1002/abio.370150307.
Turker, M., & Mavituna, F. (1987). Production of cellulase by freely suspended and immobilized cells of Trichoderma reesei. Enzyme and Microbial Technology, 9(12), 739–743. doi:10.1016/0141-0229(87)90034-2.
Wu, M.-B., Xia, L.-M., & Cen, P.-L. (2000). Studies on simultaneous production of chitosanase and chitosan degradation in situ with Trichoderma reesei in convoluted fibrous bed bioreactor. Sheng Wu Gong Cheng Xue Bao, 16(3), 368–372.
Wu, M., Xia, L., & Cen, P. (2002). Decoloring of dyes and dyeing wastewater with immobilized mycelial pellets of Coriolus versicolor. Huagong Xuebao (Chinese Edition), 53(9), 967–971.
Xia, L. M. (1998). Nuclease P1 production by immobilized Penicillium citrinum Cells. Acta Microbiologica Sinica, 38(6), 449–453.
Xia, L. M., Dai, S. M., & Cen, P. L. (1998). Saccharification of corn stover by immobilized Trichoderma reesei Cells. Acta Microbiologica Sinica, 38(2), 114–119.
Xia, L. M., Yu, S. Y., & Ceng, Z. (1993). Cellulase production by immobilized Trichoderma reesei in fed-batch fermentation. Chemistry and Industry of Forest Products, 13(3), 217–222.
Xia, L. M., Yu, S. Y., & Ding, H. W. (1994). Fermentation of corn stalk hydrolysate by the immobilized cells of Candida shehatae. Chemistry and Industry of Forest Products, 14(1), 51–55.
Mandels, M., Andreotti, R., & Roche, C. (1976). “Measurement of saccharifying cellulase.” Biotechnology and Bioengineering Symposium, 6, 21–33.
Miller, W. M., Blanch, H. W., & Wilke, C. R. (1988). A kinetic analysis of hybridoma growth and metabolism in batch and continuous suspension culture: effect of nutrient concentration, dilution rate, and pH. Biotechnology and Bioengineering, 32(8), 947–965. doi:10.1002/bit.260320803.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254. doi:10.1016/0003-2697(76)90527-3.
Acknowledgment
This project was supported by Initiative for Future Agriculture and Food System Grant no. 2001-52104-11476 from the USDA Cooperative State Research, Education, and Extension Service. The authors are grateful to Lendell Manufacturing Inc. (St. Charles, Michigan) for supplying the PU foams used in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Q., Lo, CM. & Ju, LK. Cell Immobilization with Polyurethane Foam for Retaining Trichoderma reesei Cells During Foam Fractionation for Cellulase Collection. Appl Biochem Biotechnol 156, 12–23 (2009). https://doi.org/10.1007/s12010-008-8484-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8484-9