Abstract
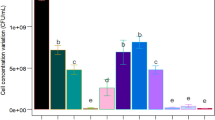
Bacillus atrophaeus’s spores are used as biological indicators to monitor sterilization processes and as a Bacillus anthracis surrogate in the development and validation of biosafety methods. The regular use of biological indicators to evaluate the efficiency of sterilization processes is a legal requirement for health services. However, its high cost hinders its widespread use. Aiming at developing a cost-effective inoculum medium, soybean molasses and nutrient-supplemented vinasse were evaluated for their effectiveness in solid-state fermentation (SSF). In biomass production, the results demonstrated that all tested compositions favor growth by providing the nutritional demands of the microorganism. Optimum casein peptone and soybean molasses concentration (1.0%, 2.5%, or 4.0%) was determined by a 2(2–0) factorial experimental design. The results have showed a positive influence of peptone on biomass production. In order to define peptone final concentration (4.0% or 6.0%), a 22 factorial experimental design was used. An optimized medium containing 4.0% soybean molasses and 4.0% casein peptone was similar in performance to a synthetic control medium (tryptone soy broth) in dry-heat thermal-resistant spore production by SSF. An experiment performed under optimum SSF conditions resulted in 1.9 × 1010 CFU g−1 dry matter with D 160 °C = 5.2 ± 0.2 min.







Similar content being viewed by others
References
Association For The Advanced Of Medical Instrumentation/AAMI (2005) ST 40:2004
Blakistone, B., Chuyate, R., Kautter, D. Jr., Charboneau, J., & Suit, K. (1999). Food Protection, 62, 262–267.
Penna, T. C., Mazzola, P. G., & Martins, A. M. (2001). BMC Infectious Diseases, 1, 16.
U.S. P. XXIX (2005). Biological indicator for dry-heat sterilization, paper strip. In: The United States Pharmacopeia. 29th rev. Rockville, MD.
Bayliss, C. E., & Waites, W. M. (1979). Journal of Applied Bacteriology, 47, 263–269.
Whitbourne, J. E., & Reich, R. R. (1979). Journal of the Parenteral Drug Association, 33, 132–143.
Gale, E. P., Pitchers, R., & Gray, P. (2002). Water Research, 36, 1640–1648.
Weber, D. J., Sickbert-Bennett, E., Gergen, M. F., & Rutala, W. A. (2003). JAMA, 289, 1274–1277.
Hodges, N. A., Melling, J., & Parker, S. J. (1980). Journal of Pharmacy and Pharmacology, 32, 126–130.
Davies, F. L., Underwood, H. M., Perkins, A. G., & Burton, H. (1977). Thermal death kinetics of Bacillus stearothermophilus spores at ultra high temperatures. 1 Laboratory determination of temperature coefficients. Journal of Food Technology, 12, 115–119.
Junge, H., Krebs, B., & Kilian, M. (2000). Pflazenschutz-Nachrichten Bayer, 1, 94–104.
Moraes, I. (2001). In: Biotecnologia Industrial. 3. LIMA et al. Ed.Blucher Ltda, São Paulo. 200–217.
Monteiro, S. M., Clemente, J. J., Henriques, A. O., Gomes, R. J., Carrondo, M. J., & Cunha, A. E. (2005). Biotechnology Progress, 21, 1026–1031.
Youkong, C., Dechmahitkul, W., & Mekvichitsaeng, P. (2004). Available from http://knowledge.biotec.or.th/doc_upload/2004113153453.doc. Accessed July 26, 2006.
Fadel, M., & Sabour, M. (2002). Journal of Biological Sciences, 2, 16–120.
Cesare-Vidaurre, T., Campos, E., & Castro-Gomez, R. J. H. (1997). Anais do VII Mexican Congress of Biotechnology and Bioengineering, Mexico.
Luna, C. L., Mariano, R. L. R., & Souto-Maior, A. M. (2002). Brazilian Journal of Chemical Engineering, 19, 133–140.
Cegla et al. (2005). United States Patent 6,913,771.
Siqueira, P. F. (2006). Master of Sciences Thesis, Federal University of Paraná/Universities of Provence and of the Mediterranean Sea, Curitiba, Brazil.
Machado, R. (1999). Master Thesis, Federal University of Rio Grande do Sul, Porto Alegre, Brazil.
Neves, L.C. M., Oliveira, K. S., Kobayashi, M. J., & Penna, T. C. V. (2006). 28th Symposium on Biotechnology for Fuels and Chemicals. Nashville, EUA.
Li, X., Yang, L., Yan, P., Zuo, F., & Jin, F. (1997). Letters in Applied Microbiology, 24, 1–4.
Sella, S. R. B. R., Vandenbergue, L. P. S., Medeiros, A. P., & Soccol, C. R. (2007). Anais XVI Simpósio Nacional de Bioprocessos—2007. Curitiba. Brazil. Cd. FES 520.
Vries, Y. P., Atmadja, R. D., Hornstra, L. M., deVos, W. M., & Abee, T. (2005). Applied and Environmental Microbiology, 71, 3248–3254.
Feavers, I. M., Foulkes, J., Setlow, B., Sun, D., Nicholson, W., Setlow, P., et al. (1990). Molecular Microbiology, 4, 275–282.
Raso, J., Barbosa-Canovas, G., & Swanson, B. G. (1998). Journal of Applied Microbiology, 85, 17–24.
Redmond, C., Baillie, L. W., Hibbs, S., & Moir, A. J. (2004). Microbiology, 150, 355–363.
Mohan, R., Chui, E. A., Biasi, L. A., & Soccol, C. R. (2005). Brazilian Archives of Biology and Technology, 48, 37–42.
Hoxey, E. V., Soper, C. J., & Davies, D. J. (1985). Journal of Applied Bacteriology, 58, 207–214.
Penna, T. C. V., Machoshvili, I. A., & Taqueda, M. E. S. (1996). PDA Journal of Pharmaceutical Science and Technology, 50, 227–237.
Olson, J. R., & Nottingham, P. M. (1980). Ecologia Microbiana de los Alimentos. Zaragosa, Acribia, p.1727.
Waites, W. M., & Bayliss, C. E. (eds.) (1980). In: Microbial growth and survival in extremes of environment. Applied bacteriology technical series. 15, 159–172. London: Academic.
USP 29 (2005). Biological Indicators for Sterilization. Available from www.pharmacopeia.cn/v29240/usp29nf24s0_c1035.html. Acessed July 25, 2007.
Association for the Advanced of Medical Instrumentation-ANSI/AAMI/ISO 11138 (1994). Sterilization of Health Care Products—Biological Indicators.
Acknowledgments
The present research was financially supported by the Secretaria de Estado da Ciência, Tecnologia e Ensino Superior—Fundo Paraná. The authors thank José Carlos Pereira Comink for the statistic revision and Neuza Araujo and Elza Retka for the laboratory support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sella, S.R.B.R., Dlugokenski, R.E.F., Guizelini, B.P. et al. Selection and Optimization of Bacillus atrophaeus Inoculum Medium and its Effect on Spore Yield and Thermal Resistance. Appl Biochem Biotechnol 151, 380–392 (2008). https://doi.org/10.1007/s12010-008-8206-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8206-3