Abstract
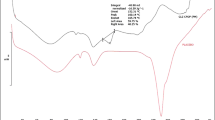
The aim of the present study was to develop pegylated cellulose acetate coating layer having leachable membrane created by controlled porosity osmotic pump (CPOP) technique. Already optimized core tablets of Captopril were used for the application of CPOP. In Box–Behnken design, cellulose acetate, polyethylene glycol, and sorbitol were used as variables and %Captopril release as a response for the coating process. Formulations were characterized by weight gain, thickness of leachable membrane, content uniformity, drug release, pH, dissolution medium osmolality effect, FTIR, and stability analysis for the optimization purpose. Optimized formulation was within the pharmacopoeial limits. Observed weight gain was 4.38–6.97%, and thickness of leachable membrane was 1.24–1.47 mm. Content uniformity was found to be 91.65–99.61%. Phosphate buffer of pH 6.8 showed 86.22–92.79% drug release and the first-order release pattern. pH-independent but osmolality-dependent drug release was observed. No incompatibility between the ingredients of prepared dosage form was observed in FTIR analysis. Accelerated stability study for 6 months showed no significant change in the prepared dosage form. Conclusively, prepared CPOP tablets can be used for the controlled release of Captopril in hypertensive patients to maintain the desired drug concentration within the body for required time period.





Similar content being viewed by others
References
Makhija, SN, Vavia, PR, “Controlled Porosity Osmotic Pump-Based Controlled Release Systems of Pseudoephedrine: I. Cellulose Acetate as a Semipermeable Membrane.” J. Control. Release, 89 (1) 5–18 (2003)
Rathbone, MJ, Hadgraft, J, Roberts, MS, Lane, ME, Modified-Release Drug Delivery Technology. CRC Press, Boca Raton (2008)
Verma, RK, Krishna, DM, Garg, S, “Formulation Aspects in the Development of Osmotically Controlled Oral Drug Delivery Systems.” J. Control. Release, 79 (1–3) 7–27 (2002)
Gupta, S, Singh, RP, Sharma, R, Kalyanwat, R, Lokwani, P, “Osmotic Pumps: A Review.” Int. J. Compr. Pharm., 2 (6) 1–8 (2011)
Keraliya, RA, Patel, C, Patel, P, Keraliya, V, Soni, TG, Patel, RC, Patel, M, “Osmotic Drug Delivery System as a Part of Modified Release Dosage Form.” ISRN pharmaceutics, 2012 (2012)
Aulton, ME, Pharmaceutics: The Science of Dosage Form Design. Churchill Livingstone, London (2000)
Aulton, ME, Taylor, KM, Aulton’s Pharmaceutics E-Book: The Design and Manufacture of Medicines. Elsevier, Amsterdam (2017)
Akhtar, MF, Hanif, M, Majeed, A, Shah, S, “Formulation Development and Optimization of Captopril Containing Tablets Through Box-Behnken Design.” Latin Am. J. Pharm., 37 (7) 1414–1423 (2018)
Abbas, G, Hanif, M, Khan, MA, “pH Responsive Alginate Polymeric Rafts for Controlled Drug Release by Using Box Behnken Response Surface Design.” Des. Monomers Polym., 20 (1) 1–9 (2017)
Anju, CL, Palanichamy, S, Rajesh, M, Ramasubramaniyan, P, Solairaj, P, “Formulation and Evaluation of Osmotic Drug Delivery System of Ibuprofen.” Ars Pharm., 55 (4) 44–51 (2014)
Pharmacopoeia, B, “Specific Monograph: British Pharmacopoeia Commission.” London (2015)
Qazi, F, Shoaib, MH, Yousuf, RI, Qazi, TM, Mehmood, ZA, Hasan, SMF, “Formulation Development and Evaluation of Diltiazem HCl Sustained Release Matrix Tablets Using HPMC K4 M and K100M.” Pak. J. Pharm. Sci., 26 (4) 653–663 (2013)
Costa, P, Lobo, JMS, “Modeling and Comparison of Dissolution Profiles.” Eur. J. Pharm. Sci., 13 (2) 123–133 (2001)
Ahmed, K, Shoaib, MH, Yousuf, RI, Qazi, F, Anwer, S, Nasiri, MI and Mahmood, ZA, “Use of Opadry® CA—A Cellulose Acetate/Polyethylene Glycol System for Rate‐Controlled Osmotic Drug Delivery of Highly Soluble Antispastic Agent Eperisone HC l.” Adv. Polym. Tech., 37 (8) 2730–2742 (2018)
Pharmacopeia, U, “USP 39 NF 34.” (2015)
McClelland, GA, Sutton, SC, Engle, K, Zentner, GM, “The Solubility-Modulated Osmotic Pump: In Vitro/In Vivo Release of Diltiazem Hydrochloride.” Pharm. Res., 8 (1) 88–92 (1991)
Zentner, GM, McClelland, GA, Sutton, SC, “Controlled Porosity Solubility- and Resin-Modulated Osmotic Drug Delivery Systems for Release of Diltiazem Hydrochloride.” J. Control. Release, 16 (1–2) 237–243 (1991)
Rapolu, K, Sanka, K, Vemula, PK, Aatipamula, V, Mohd, AB, Diwan, PV, “Optimization and Characterization of Gastroretentive Floating Drug Delivery System Using Box-Behnken Design.” Drug Dev. Ind. Pharm., 39 (12) 1928–1935 (2013)
Thakkar, HP, Pancholi, N, Patel, CV, “Development and Evaluation of a Once-Daily Controlled Porosity Osmotic Pump of Tapentadol Hydrochloride.” AAPS PharmSciTech, 17 (5) 1248–1260 (2016)
Theeuwes, F, “Elementary Osmotic Pump.” J. Pharm. Sci., 64 (12) 1987–1991 (1975)
Santus, G, Baker, RW, “Osmotic Drug Delivery: A Review of the Patent Literature.” J. Control. Release, 35 (1) 1–21 (1995)
Shaikh, SF, Mane, RS, Min, BK, Hwang, YJ, Joo, O, “D-Sorbitol-Induced Phase Control of TiO2 Nanoparticles and Its Application for Dye-Sensitized Solar Cells.” Sci. Rep., 6 20103 (2016)
Chen, C, Liu, W, Wang, Z, Peng, K, Pan, W, Xie, Q, “Novel Form Stable Phase Change Materials Based on the Composites of Polyethylene Glycol/Polymeric Solid-Solid Phase Change Material.” Sol. Energy Mater. Sol. Cells, 134 80–88 (2015)
Zhijiang, C, Yi, X, Haizheng, Y, Jia, J, Liu, Y, “Poly (Hydroxybutyrate)/Cellulose Acetate Blend Nanofiber Scaffolds: Preparation, Characterization and Cytocompatibility.” Mater. Sci. Eng., C, 58 757–767 (2016)
Asif, U, Sherwani, AK, Akhtar, N, Shoaib, MH, Hanif, M, Qadir, MI, Zaman, M, “Formulation Development and Optimization of Febuxostat Tablets by Direct Compression Method.” Adv. Polym. Technol., 35 (2) 129–135 (2016)
Acknowledgment
Authors are very much thankful to the department of research and development of Bahauddin Zakariya University Multan, Pakistan, and Higher Education Commission (HEC), Pakistan, for providing the financial grant and Department of Pharmaceutics, Bahauddin Zakariya University Multan, Pakistan, for providing laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akhtar, M.F., Hanif, M. Leachable pegylated cellulose acetate complex: a promising approach for controlled porosity osmotic pump tablets of Captopril. J Coat Technol Res 17, 439–446 (2020). https://doi.org/10.1007/s11998-019-00290-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-019-00290-7