Abstract
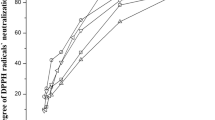
The aim of this study was to evaluate the replacement aspects of conventional methods (petroleum-based solvent and Folch assay) by alternative methods (bio-based and biodegradable solvent 2-methyltetrahydrofuran (MeTHF) and supercritical CO2 (SC-CO2)) for seed oil extraction from anise (Pimpinella anisum L.) and fennel (Foeniculum vulgare Mill.). Results showed that the highest oil yield of aniseeds was obtained by using Folch (24.07%) and MeTHF (23.65%) extraction methods whereas fennel seeds had 20.02% and 18.72%, respectively. Fatty acid composition of both seed oils obtained by the two green extraction methods was similar to the conventional ones with the predominance of petroselinic acid (54.22–61.25% in fennel and 42.39–48.97% in anise). Besides, SC-CO2 method allowed to obtain the maximum of sterol content in anise (3.85 mg/g of oil) and fennel (4.64 mg/g of oil) seed oils. Furthermore, anise and fennel seed oils extracted with MeTHF method significantly showed higher total phenolic content (2.43 and 1.32 mg GA/g oil, respectively), stronger antioxidant activity (9.23 and 5.04 μmol TEAC/g oil, respectively), and oxidative stability (8.23 and 10.15 h, respectively) than the other methods (p < 0.05). In conclusion, MeTHF appeared to be a good substitute to petroleum solvents for recovery of high oil quality from Pimpinella anisum and Foeniculum vulgare seeds.


Similar content being viewed by others
References
Abert Vian, M., Dejoye Tanzi, C., & Chemat, F. (2013). Techniques conventionelles et innovantes, et solvants alternatifs pour l’extraction des lipides de micro-organismes. Oilseeds fats Crop. Lipids, 20, 1–6.
Akinoso, R., & Adeyanju, J. A. (2012). Optimization of edible oil extraction from ofada rice bran using response surface methodology. Food and Bioprocess Technology, 5(4), 1372–1378.
Anonymos (1997). Determination of the composition of the sterol fraction of animal and vegetable oils and fats by TLC and capillary GLC. AOCS, Official Method Ch 6–91.
Ben Khedir, S., Bardaa, S., Chabchoub, N., Moalla, D., Sahnoun, Z., & Rebai, T. (2017). The healing effect of Pistacia lentiscus fruit oil on laser burn. Pharmaceutical Biology, 55(1), 1407–1414.
Bernardo-Gil, M. G., Grenha, J., Santos, J., & Cardoso, P. (2002). Supercritical fluid extraction and characterization of oil from hazelnut. European Journal of Lipid Science and Technology, 104(7), 402–409.
Bettaieb Rebey, I., Rahali, F. Z., Saidani Tounsi, M., Marzouk, B., & Ksouri, R. (2016). Variation in fatty acid and essential oil composition of sweet fennel (Foeniculum vulgare Mill) seeds as affected by salinity. Journal of New Sciences, Agriculture and Biotechnology, IABC, 6, 1233–1240.
Bettaieb Rebey, I., Bourgou, S., Saidani Tounsi, M., Fauconnier, M. L., & Ksouri, R. (2018). Comparative assessment of phytochemical profiles and antioxidant properties of Tunisian and Egyptian anise (Pimpinella anisum L.) seeds. Plant Biosystems, 152(5), 971–978.
Boutin, O., & Badens, E. (2009). Extraction from oleaginous seeds using supercritical CO2: experimental design and products quality. Journal of Food Engineering, 92(4), 396–402.
Breil, C., Meullemiestre, A., Vian, M., & Chemat, F. (2016). Bio-based solvents for green extraction of lipids from oleaginous yeast biomass for sustainable aviation biofuel. Molecules, 21, E196.
Cecchi, G., Biasini, S., & Castano, J. (1985). Méthanolyse rapide des huiles en solvant. Note de laboratoire. Revue Française des Corps Gras, 4, 163–164.
Chemat, F., Rombaut, N., Sicaire, A. G., Meullemiestre, A., Fabiano-Tixier, A. S., & Abert-Vian, M. (2017). Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrasonics Sonochemistry, 34, 540–560.
Cheung, P. C. K., Leung, A. Y. H., & Ang, P. O. (1998). Comparison of supercritical carbon dioxide and Soxhlet extraction of brown seaweed Sargassum hemiphyllum (turn.). Journal of Agricultural and Food Chemistry, 46(10), 4228–4232.
Danh, L. T., Han, L. N., Triet, N. D. A., Zhao, J., Mammucari, R., & Foster, N. (2013). Comparison of chemical composition, antioxidant and antimicrobial activity of lavender (Lavandula angustifolia L.) essential oils extracted by supercritical CO2, hexane and hydrodistillation. Food and Bioprocess Technology, 6(12), 3481–3489.
Dong, S., Zhang, R., Ji, Y. C., Hao, J. Y., Ma, W. W., Chen, X. D., & Yu, H. L. (2016). Soy milk powder supplemented with phytosterol esters reduced serum cholesterol level in hypercholesterolemia independently of lipoprotein E genotype: a random clinical placebo-controlled trial. Nutrition Research, 36(8), 879–884.
Folch, J., Lees, M., & Stanley, G. H. S. (1957). A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry, 226, 497–509.
Holser, R. (2003). Seed conditioning and meadow foam press oil quality. Industrial Crops and Products, 17(1), 23–26.
Kiralan, M., Bayrak, A., & Mucahit, T. O. (2009). Oxidation stability of virgin olive oils from some important cultivars in East Mediterranean area in Turkey. Journal of the American Oil Chemists' Society, 86(3), 247–252.
Konuskan, D. B., Kamiloglu, O., & Demirkeser, Ö. (2019). Fatty acid composition, total phenolic content and antioxidant activity of grape seed oils obtained by cold- pressed and solvent extraction. Indian Journal of Pharmaceutical Education and Research, 53(1), 144–150.
Koubaa, M., Roselló-Soto, E., Šic Žlabur, J., Režek Jambrak, A., Brnčić, M., Grimi, N., Boussetta, N., & Barba, F. J. (2015). Current and new insights in the sustainable and green recovery of nutritionally valuable compounds from Stevia rebaudiana Bertoni. Journal of Agricultural and Food Chemistry, 63(31), 6835–6846.
Koubaa, M., Mhemdi, H., Barba, F. J., Roohinejad, S., Greiner, R., & Vorobiev, E. (2016). Oilseed treatment by ultrasounds and microwaves to improve oil yield and quality: an overview. Food Research International, 85, 59–66.
Koubaa, M., Mhemdi, H., Barba, F. J., Angelotti, A., Bouaziz, F., Chaabouni, S. E., & Vorobiev, E. (2017). Seed oil extraction from red prickly pear using hexane and supercritical CO2: assessment of phenolic compound composition, antioxidant and antibacterial activities. Journal of the Sciences and Food Agricultural, 97(2), 613–620.
Kozłowska, M., Gruczyńska, E., Ścibisz, I., & Rudzińska, M. (2016). Fatty acids and sterols composition, and antioxidant activity of oils extracted from plant seeds. Food Chemistry, 213, 450–456.
Kulkarni, N. G., Kar, J. R., & Singhal, R. S. (2017). Extraction of flaxseed oil: a comparative study of three-phase partitioning and supercritical carbon dioxide using response surface methodology. Food and Bioprocess Technology, 10(5), 1–9.
Liu, S. X., & Mamidipally, P. K. (2005). Quality comparison of rice bran oil extracted with limonene and hexane. Cereal Chemistry, 82(2), 209–215.
Liu, G., Xu, X., Hao, Q. F., & Gao, Y. X. (2009). Supercritical CO2 extraction optimization of pomegranate (Punica granatum L.) seed oil using response surface methodology. LWT - Food Science and Technology, 42(9), 1491–1495.
Liu, C., Han, X., Cai, L., Lu, X., Han, X. X., & Ying, T. J. (2012). Effect of postharvest UV-C irradiation on phenolic compound content and antioxidant activity of tomato fruit during storage. Journal of Integrative Agriculture, 11(1), 159–165.
Malacrida, C. R., & Jorge, N. (2012). Yellow passion fruit seed oil (Passiflora edulis f. flavicarpa): physical and chemical characteristics. Brazilian Archives of Biology and Technology, 55(1), 127–134.
Malheiro, R., Casal, S., Teixeira, H., Bento, A., & Pereira, J. A. (2013). Effect of olive leaves addition during the extraction process of overmature fruits on olive oil quality. Food and Bioprocess Technology, 6(2), 509–521.
Mariod, A. A., Matthaȕs, B., & Ismail, M. (2011). Comparison of supercritical fluid and hexane extraction methods in extracting kenaf (Hibiscus cannabinus) seed oil lipids. Journal of the American Oil Chemists' Society, 88(7), 931–935.
Mhemdi, H., Rodier, E., Kechaou, N., & Fages, J. (2011). A supercritical tuneable process for the selective extraction of fats and essential oil from coriander seeds. Journal of Food Engineering, 105(4), 609–616.
Miguel, M. G., Nunes, S., Dandlen, S. A., Cavaco, A. M., & Antunes, M. D. (2010). Phenols and antioxidant activity of hydro-alcoholic extracts of própolis from Algarve, south of Portugal. Food and Chemical Toxicology, 48(12), 3418–3423.
Misirli, H., Domac, F. M., Somay, G., Araal, O., Ozer, B., & Adiguzel, T. (2008). N-hexane induced polyneuropathy: a clinical and electrophysiological follow up. Electroencephalography and Clinical Neurophysiology, 48, 103–108.
Molero Gómez, A., & Martı́nez de la Ossa, E. (2002). Quality of borage seed oil extracted by liquid and supercritical carbon dioxide. Chemical Engineering Journal, 88(1-3), 103–109.
Montedoro, G., Servili, M., Baldioli, M., & Miniati, E. (1992). Simple and hydrolyzable phenolic compounds in virgin olive oil. 1. Their extraction, separation, and quantitative and semiquantitative evaluation by HPLC. Journal of Agricultural and Food Chemistry, 40(9), 1571–1576.
Moura, L. S., Carvalho, R. N., Stefanini, M. B., Ming , L. C., & Meireles, M. A. A. (2005). Supercritical fluid extraction from fennel (Foeciculum vulgarae): global yield, composition and kinetic data. Journal of Supercritical Fluids, 35(3), 212–19.
Nyam, K. L., Tan, C. P., Lai, O. M., Long, K., & Man, Y. B. C. (2011). Optimization of supercritical CO2 extraction of phytosterol-enriched oil from Kalahari melon seeds. Food and Bioprocess Technology, 4(8), 1432–1441.
Pereira, C. G., Angela, M., & Meireles, A. (2010). Supercritical fluid extraction of bioactive compounds: fundamentals, applications and economic perspectives. Food and Bioprocess Technology, 3(3), 340–372.
Pereira, M. G., Hamerskia, F., Andradeb, E. F., Scheera, A. P., & Corazzaa, M. L. (2017). Assessment of subcritical propane, ultrasound-assisted and Soxhlet extraction of oil from sweet passion fruit (Passiflora alata Curtis) seeds. The Journal of Supercritical Fluids, 128, 338–348.
Ramadan, M. F., & Moersel, J. T. (2006). Screening of the radical action of vegetable oils. Journal of Food Composition and Analysis, 19(8), 838–842.
Ramadan, M. F., Asker, M. M. S., & Tadros, M. (2012). Antiradical and antimicrobial properties of cold-pressed black cumin and cumin oils. European Food Research and Technology, 234(5), 833–844.
Ribas, S. A., Sichieri, R., Moreira, A. S. B., Souza, D. O., Cabral, C. T. F., Gianinni, D. T., & Cunha, D. B. (2017). Phytosterol-enriched milk lowers LDL-cholesterol levels in Brazilian children and adolescents: double-blind, cross-over trial. Nutrition, Metabolism, and Cardiovascular Diseases, 27, 971–977.
Salgın, U., Salgın, S., Din, D., Ekici, D. D., & Uludag, G. (2016). Oil recovery in rosehip seeds from food plant waste products using supercritical CO2 extraction. Journal of Supercritical Fluids, 118, 194–202.
Sayed Ahmad, B., Talou, T., Saad, Z., Hijazi, A., Cerny, M., Kanaan, H., Chokr, A., & Merah, O. (2018). Fennel oil and by-products seed characterization and their potential applications. Industrial Crops and Products, 111, 92–98.
Shokri, A., Hatami, T., & Khamforoush, M. (2011). Near critical carbon dioxide extraction of anise (Pimpinella Anisum L.) seed: Mathematical and artificial neural network modeling. Journal of Supercritical Fluids, 58(1), 49–57.
Sicaire, A. G., Vian, M. A., Fine, F., Carré, P., Tostain, S., & Chemat, F. (2015). Experimental approach versus COSMO-RS assisted solvent screening for predicting the solubility of rapeseed oil. Oilseeds & fats Crops and Lipids, 22, 1–7.
Simándi, B., Deák, A., Rónyai, E., Yanxiang, G., Veress, T., Lemberkovics, E., Then, M., Sass-Kiss, A., & Vámos-Falusi, Z. (1999). Supercritical carbon dioxide extraction and fractionation of fennel oil. Journal of Agricultural and Food Chemistry, 47(4), 1635–1640.
Sovilj, M. N. (2010). Critical review of supercritical carbon dioxide extraction of selected oil seeds. Acta Periodica technologica, 41, 1–203.
Stupp, T., Freitas, R. A., Sierakowski, M. R., Deschamps, F. C., Wisniewski, A., & Biavatti, M. W. (2008). Characterization and potential uses of Copaifera langsdorfii seeds and seed oil. Bioresource Technology, 99(7), 2659–2663.
Tabee, E., Azadmard-Damirchi, S., Jägerstad, M., & Dutta, P. C. (2008). Lipids and phytosterol oxidation in commercial French fries commonly consumed in Sweden. Journal of Food Composition and Analysis, 21(2), 169–177.
Tasioula-Margari, M., & Tsabolatidou, E. (2015). Extraction, separation, and identification of phenolic compounds in virgin olive oil by HPLC-DAD and HPLC-MS. Antioxidants, 4(3), 548–562.
Virot, M. V., Tomao, C., Ginies, F., Visinoni, & Chemat, F. (2008). Microwave-integrated extraction of total fats and oils. Journal of Chromatography A, 1196, 147–152.
Funding
This work was supported by the Tunisian Ministry of Higher Education and Scientific Research (LR15CBBC06).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bettaieb Rebey, I., Bourgou, S., Detry, P. et al. Green Extraction of Fennel and Anise Edible Oils Using Bio-Based Solvent and Supercritical Fluid: Assessment of Chemical Composition, Antioxidant Property, and Oxidative Stability. Food Bioprocess Technol 12, 1798–1807 (2019). https://doi.org/10.1007/s11947-019-02341-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-019-02341-8