Abstract
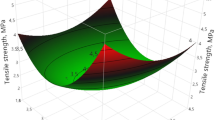
The effectiveness of using a tapioca starch–glycerol matrix containing natamycin to control Saccharomyces cerevisiae activity in a model system was studied and the effect of the formulation on physico-chemical properties was also evaluated. The presence of natamycin tended to depress firmness at break and Young modulus and to increase strain at break. Colour was also affected by antimycotic presence. The importance of these changes will be determined by the characteristics of the product to which the antimicrobial film will be applied. The films developed were capable of acting as a hurdle against S. cerevisiae in food systems during storage: they acted as an effective reservoir of the antimycotic which was also available to prevent an external contamination. The films containing 1.85 mg natamycin/dm2 of natamycin developed a fungistatic effect till 72 h of storage, while those with a 3.70-mg natamycin/dm2 concentration developed a fungicidal action allowing the selection of the proper formulation according to the antimicrobial goal pursuit. As natamycin addition affects mechanical properties and colour of the films, it is advisable to use the lower natamycin concentration that allows the attainment of the goal pursued for film application.





Similar content being viewed by others
References
Arzate-Vázquez, I., Chanona-Pérez, J. J., Calderón-Domínguez, G., Terres-Rojas, E., Garibay-Febles, V., Martínez-Rivas, A., & Gutiérrez-López, G. F. (2012). Microstructural characterization of chitosan and alginate films by microscopy techniques and texture image analysis. Carbohydrate Polymers, 87, 289–299.
ASTM E1925. (1995). Standard test method for yellowness index of plastics. Philadelphia: American Society for Testing and Materials.
ASTM E96-00. (2000). Standard test method for water vapor transmission of materials. Philadelphia: American Society for Testing and Materials.
Basch C, Carpenco J, Jagus R & Flores S (2011) Individual and combined performance of nisin and potassium sorbate supported in tapioca strach edible fims. Proceedings of the 11th. International Congress on Engineering and Food (ICEF 11), 2, 979–980. ISBN: 978-960-89789-4-2. SET ISBN: 978–96.
Baumgartner, S., Lahajnar, G., Sepe, A., & Kristl, J. (2005). Quantitative evaluation of polymer concentration profile during swelling of hydrophilic matrix tablets using H NMR and MRI methods. Journal of Pharmaceutics and Biopharmaceutics, 59, 299–306.
Chen, M. H., Yeh, G. H., & Chiang, B. H. (1996). Antimicrobial and physicochemical properties of methylcellulose and chitosan films containing a preservative. Journal of Food Processing and Preservation, 20, 379–390.
Cong, F. S., Zhang, Y. G., & Dong, W. Y. (2007). Use of surface coatings with natamycin to improve the storability of Hami melon at ambient temperature. Postharvest Biology and Technology, 46(1), 71–75.
de Oliveira, T., Soares, N., Pereira, R., & Fraga, K. (2007). Development and evaluation of antimicrobial natamycin-incorporated film in gorgonzola cheese preservation. Food Packaging and Technology, 20, 423–432.
Dos Santos Pires, A. C., De Ferreira Soares, N. F., De Andrade, N. J., Mendes Da Silva, L. H., Peruch Camilloto, G., & Campos Bernardes, P. (2008). Development and evaluation of active packaging for sliced mozzarella preservation. Packaging Technology and Science, 21(7), 375–383.
El-Diasty, E., El-Kaseh, R., & Salem, R. (2009). The effect of natamycin on keeping quality and organoleptic characters of yoghurt. Arab Journal of Biotechnology, 12(1), 41–48.
Fajardo, P., Martins, J. T., Fuciños, C., Pastrana, L., Teixeira, J. A., & Vicente, A. A. (2010). Evaluation of a chitosan-based edible film as carrier of natamycin to improve the storability of Saloio cheese. Journal of Food Engineering, 101(4), 349–356.
Flores, S. K., Famá, L., Rojas, A. M., Goyanes, S., & Gerschenson, L. (2007). Physical properties of tapioca-starch edible films. Influence of filmmaking and potassium sorbate. Food Research International, 40(2), 257–265.
Flores, S. K., Haedo, A., & Campos, C. (2007). Antimicrobial performance of potassium sorbate supperted in tapioca starch edible films. Eur Food Res Technology, 225, 375–384.
Flores SK, Gerschenson LN, Jagus RJ & Sanjurjo KJ (2011) Strategies for Extending Shelf Life of Foods Using Antimicrobial Edible Films. Focus on Food Engineering (ISBN: 978-1-61209-895-1). Editor: Robert J. Shreck. Editorial: Nova Science Publishers, Inc. Hauppauge, NY, USA. Chapter 2, 69–99
Franssen, L. R., Rumsey, T. R., & Krochta, J. M. (2004). Whey protein film composition effects on potassium sorbate and natamycin diffusion. Journal of Food Science, 69(5), 347–350.
Gallo, L. I., & Jagus, R. J. (2007). Modelling Saccharomyces cerevisiae inactivation by natamycin in liquid cheese whey. Brazilian Journal of Food Technology, 9, 311–316.
Gennadios, A., Weller, C., & Gooding, C. (1994). Measurement errors in water vapor permeability of highly permeable, hydrophilic edible films. Journal of Food Engineering, 21, 395–409.
Ghanbarzadeha, B., & Oromiehib, A. R. (2008). Biodegradable biocomposite films based on whey protein and zein: barrier, mechanical properties and AFM analysis. International Journal of Biological Macromolecules, 43, 209–215.
Gontard, N., Guilbert, S., & Cuq, J.-L. (1992). Edible wheat gluten films: influence variables on film properties using methodology of the main process response surface. Journal of Food Science, 57(1), 190–195.
Gould, G. (1997). Methods of preservation and extension of shelf life. International Journal of Food Microbiology, 33(1), 51–64.
Hanušová, K., Šťastná, M., Votavová, L., Klaudisová, K., Dobiáš, J., Voldřich, M., & Marek, M. (2010). Polymer films releasing nisin and/or natamycin from polyvinyldichloride lacquer coating: nisin and natamycin migration, efficiency in cheese packaging. Journal of Food Engineering, 99(4), 491–496.
Hill B, Roger T & Vorhagen FW (1997) Comparative analysis of the quantization of color spaces on the basis of the CIELAB color-difference formula. ACM Transactions on Graphics, (16), No. 2.
Horcas, I., Fernandez, R., Gomez-Rodriguez, J. M., & Colchero, J. (2007). WSXM: a software for scanning probe microscopy and a tool for nanotechnology. Review of Scientific Instruments, 78(1), 013705.
Koontz, J. L., Marcy, J. E., Barbeau, W. E., & Duncan, S. E. (2003). Stability of natamycin and its cyclodextrin inclusion complexes in aqueous solution. Journal of Agricultural and Food Chemistry, 51(24), 7111–7114.
Kristo, E., Koutsoumanis, K., & Biliaderis, C. (2008). Thermal, mechanical and water vapor barrier properties of sodium caseinate films containing antimicrobials and their inhibitory action on Listeria monocytogenes. Food Hidrocolloids, 22, 373–386.
Mano, J., & Viana, J. (2001). Effects of the strain rate and temperature in stress–strain tests: study of the glass transition of a polyamide-6. Polymer testing, 20, 937–943.
Pintado, C. M. B. S., Ferreira, M. A. S. S., & Sousa, I. (2010). Control of pathogenic and spoilage microorganisms from cheese surface by whey protein films containing malic acid, nisin and natamycin. Food Control, 21(3), 240–246. Elsevier.
Pranoto, Y., Rakshit, S. K., & Salokhe, V. M. (2005). Enhancing antimicrobial activity of chitosan films by incorporating garlic oil, potassium sorbate and nisin. LWT Food Science and Technology, 38, 859–865.
Ramos, O. L., Silva, S. I., Soaresa, J. C., Fernandesa, J. C., Poçasa, M. F., Pintadoa, M. E., & Malcata, F. X. (2012). Features and performance of edible films, obtained from whey protein isolate formulated with antimicrobial compounds. Food Research International, 45(1), 351–361.
Sanjurjo, K., Flores, S., Gerschenson, L., & Jagus, R. J. (2006). Study of the performance of nisin supported in edible films. Food Research International, 39(6), 749–754.
Sebti, I., Blanc, D., Carnet-Ripochem, A., Saurel, R., & Coma, V. (2004). Experimental study and modeling of nisin diffusion in agarose gels. Journal of Food Engineering, 63, 185–190.
Sokal, R., & Rohlf, J. (2000). Biometry. The principles and practice of statistics in biological research. San Francisco: W. H. Freeman and Company.
te Welscher, Y. M., Ten Napel, H. H., Balagué, M. M., Souza, C. M., Riezman, H., De Kruijff, B., & Breukink, E. (2008). Natamycin blocks fungal growth by binding specifically to ergosterol without permeabilizing the membrane. The Journal of Biological Chemistry, 283(10), 6393–6401.
te Welscher, Y. M., Jones, L., van Leeuwen, M. R., Dijksterhuis, J., De Kruijff, B., Eitzen, G., & Breukink, E. (2010). Natamycin inhibits vacuole fusion at the priming phase via a specific interaction with ergosterol. Antimicrobial Agents and Chemotherapy, 54(6), 2618–2625.
Thomas, A. H. (1976). Analysis and assay of polyene antifungal antibiotics. The Analyst, 101, 321–339.
Trezza, T., & Krochta, J. (2000). Color stability of edible coatings during prolonged storage. Journal of Food Science, 65(1), 1166–1169.
Ture, H., Eroglu, E., Ozen, B., & Soyer, F. (2011). Effect of biopolymers containing natamycin against Aspergillus niger and Penicillium roquefortii on fresh kashar cheese. International Journal of Food Science Technology, 46(1), 154–160.
Vásconez, M. B., Flores, S. K., Campos, C. A., Alvarado, J., & Gerschenson, L. N. (2009). Antimicrobial activity and physical properties of chitosan–tapioca starch based edible films and coatings. Food Research International, 42(7), 762–769.
Acknowledgements
This study was financially supported by University of Buenos Aires (UBACyT 20020100100125), National Agency of Scientific and Technical Research (PICT 1172), and CONICET (PIP 531). The authors also wish to thank Industrias del Maíz S.A. (Argentina), Mallickrodt (Argentina) and DSM (Argentina).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ollé Resa, C.P., Gerschenson, L.N. & Jagus, R.J. Effect of Natamycin on Physical Properties of Starch Edible Films and Their Effect on Saccharomyces cerevisiae Activity. Food Bioprocess Technol 6, 3124–3133 (2013). https://doi.org/10.1007/s11947-012-0960-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-012-0960-0