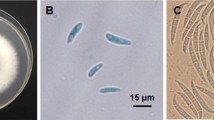
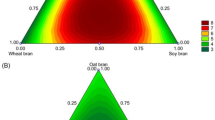
Abstract
The production of cutinase by solid-state fermentation, using by-products such as wheat bran, rice bran, or soybean rind, was carried out using a Fusarium oxysporum strain. The fermentation process was optimized using a central composite design. The best conditions for cutinase production were achieved at 28–30 °C, with water added at 100–150% (w/w) after 72 h of incubation, in the range of 11.7–15.5 U/mL. In addition, the resolution of (R,S)-2-octanol and (R,S)-ibuprofen was performed to evaluate the enantioselectivity of the preparations of cutinase. The cutinase produced from the soybean rind attained higher yields and enantioselectivity in the resolution of (R,S)-2-octanol with octanoic acid in isooctane (E = 9.6). For the (R,S)-ibuprofen resolution, the cutinase produced from rice bran reached the best yields (E = 5.6). This work demonstrated that the enzymes can be produced from different media, such as from by-products or residues rich in carbon sources that do not necessarily present the same biochemical properties, which may be useful for industrial applications.


Similar content being viewed by others
References
Adeniran, H. A., Abiose, S. H., & Ogunsua, A. O. (2009). Production of fungal B amylase and amyloglucosidase on some Nigerian agricultural residues. Food and Bioprocess Technology, doi:10.1007/s11947-008-0141-3.
Benjamim, S., & Pandey, A. (2000). Isolation and characterization of three distinct forms of lipases from Candida rugosa produced in solid state fermentation. Brazilian Archives of Biology and Technology, 43, 453–460.
Borreguero, I., Carvalho, C. M. L., Cabral, J. M. S., Sinisterra, J. V., & Alcantara, A. R. (2001). Enantioselective properties of Fusarium solani pisi cutinase on transesterification of acyclic diols: activity and stability evaluation. Journal of Molecular Catalysis B: Enzymatic, 11, 613–622.
Brandelli, A. (2008). Bacterial Keratinases: Useful Enzymes for Bioprocessing Agroindustrial Wastes and Beyond. Food and Bioprocess Technology, 1, 105–116.
Calado, C. R. C., Monteiro, S. M. S., Cabral, J. M. S., & Fonseca, L. P. (2002). Effect of pre-fermentation on the production of cutinase by a recombinant Saccharomyces cerevisiae. Journal of Bioscience and Bioengineering, 93, 354–359.
Cardenas, F., Castro-Alvarez, M. S., Sanchez-Monteiro, J. M. S., Sanchez, A., Sinisterra, J. V., Valmaseda, M., et al. (2001). Novel microbial lipases: catalytic activity in reactions in organic media. Enzyme and Microbial Technology, 28, 145–154.
Carvalho, C. M. L., Aires-Barros, M. R., & Cabral, J. M. S. (1999). Cutinase: from molecular level to bioprocess development. Biotechnology and Bioengineering, 66, 17–34.
Carvalho, P. O., Contesini, F. J., Bizaco, R., Calaffati, S. A., & Macedo, G. A. (2006). Optimization of enantioselective resolution of racemic ibuprofen by native lipase from Aspergillus niger. Journal of Industrial Microbiology and Biotechnology, 33, 713–718.
Chen, C.-S., Fujimoto, Y., Girdaukas, G., & Sih, G. J. (1982). Quantitative analysis of biochemical kinetic resolutions enantiomers. Journal of the American Chemical Society, 104, 7294–7299.
Faber, K. (2000). Biotransformation in organic chemistry. Berlin: Springer.
Garcia-Garza, J. A., & Fravel, D. R. (1998). Effect of relative humidity on sporulation of Fusarium oxysporum in various formulations and effect of water on spore movement through soil. Biological Control, 88, 544–549.
Gonçalves, A. M., Aires-Barros, M. R., & Cabral, J. M. S. (2003). Interaction of an anionic surfactant with a recombinant cutinase from Fusarium solani pisi: a spectroscopy study. Enzyme and Microbial Technology, 32, 868–879.
Griffin, D. H. (1996). Fungal physiology (p. 472). New York: Wiley.
Han, B., Ma, Y., Rombouts, F. M., & Nout, M. J. R. (2003). Effects of temperature and relative humidity on growth and enzyme production by Actinomucor elegans and Rhizopus oligosporus during sufu pehtze preparation. Food Chemistry, 81, 27–34.
Holker, U., Hofer, M., & Lenz, J. (2004). Biotechnological advantages of laboratory-scale solid state fermentation with fungi. Applied Microbiology and Biotechnology, 64, 175–186.
Hornby, J. M., Jacobitz-Kizzier, S. M., McNeel, D. J., Jensen, E. C., Treves, D. S., & Nickerson, K. W. (2004). Inoculum size effect in dimorphic fungi: extracellular control of yeast-mycelium dimorphism in Ceratocystis ulmi. Applied and Environmental Microbiology, 70, 1356–1359.
Kulkarni, R. K., & Nickerson, K. W. (1981). Nutritional control of dimorphism in Ceratocystis ulmi. Experimental Mycology, 5, 148–154.
Longhi, S., & Cambillau, C. (1999). Structure-activity of cutinase, a small lipolytic enzyme. Biochemistry and Biophysics Acta, 144, 185–196.
Lu, W., Li, D., & Wu, Y. (2003). Influence of water activity and temperature on xylanase biosynthesis in pilot-scale solid-state fermentation by Aspergillus sulphureus. Enzyme and Microbial Technology, 32, 305–311.
Macedo, G. A., & Pio, T. F. (2005). A rapid screening method for cutinase producing microorganisms. Brazilian Journal of Microbiology, 36, 338–394.
Mannesse, M. L. M., Cox, R. C., Koops, B. C., Verheij, H. M., Haas, G. H., Egmong, M., et al. (1995). Cutinase from Fusarium solani pisi hydrolyzing triglyceride analogues. Effect of acyl chain length and position in the substrate molecule on activity and enantioselectivity. Biochemistry, 34, 6400–6407.
Mustranta, A. (1992). Use of lipase in the resolution of racemic ibuprofen. Applied Microbiology and Biotechnology, 38, 61–66.
Pio, T. F., & Macedo, G. A. (2007). Optimizing the production of cutinase by Fusarium oxysporum using response surface methodology. Journal of Industrial Microbiology and Biotechnology, 10, 101–111.
Robinson, T., Singh, D., & Nigan, P. (2001). Solid-state fermentation: a promising microbial technology for secondary metabolite production. Applied Microbiology and Biotechnology, 55, 284–289.
Rodriguez, J. A., Mateos, J. C., Nungaray, J., González, V., Bhagnagar, T., Roussos, S., et al. (2006). Improving lipase production by nutrient source modification using Rhizopus homothallicus cultured in solid state fermentation. Process Biochemistry, 41, 2264–2269.
Santos, M. M., Rosa, A. S., Dal’Boit, S., Mitchell, D. A., & Krieger, N. (2004). Thermal denaturation: is solid-state fermentation really a good technology for the production of enzymes? Bioresource Technology, 93, 261–268.
Suryanarayan, S. (2003). Current industrial practice in solid state fermentation for secondary metabolite production: the Biocon India experience. Biochemical Engineering Journal, 13, 189–195.
Szendefy, J., Szakacs, G., & Christopher, L. (2006). Potential of solid-state fermentation enzymes of Aspergillus oryzae in biobleaching of paper pulp. Enzyme and Microbial Technology, 39, 1354–1360.
Treichel, D., Oliveira, D., Mazutti, M. A., Di Luccio, M., & Oliveira, J. V. (2009). A review on microbial lipases production. Food and Bioprocess Technology, doi:10.1007/s11947-009-0202-2.
Tyagi, S., & Pleiss, J. (2006). Biochemical profiling in silico—predicting substrate specificities of large enzyme families. Journal of Biotechnology, 124, 108–116.
Viniegra-Gonzalez, G., Favela-Torres, E., Aguilar, C. N., Romero-Gomez, S. D., Diaz-Godinez, G., & Augur, C. (2003). Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochemical Engineering Journal, 13, 157–167.
Acknowledgments
The authors wish to thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for their financial support and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fraga, L.P., Carvalho, P.O. & Macedo, G.A. Production of Cutinase by Fusarium oxysporum on Brazilian Agricultural By-products and its Enantioselective Properties. Food Bioprocess Technol 5, 138–146 (2012). https://doi.org/10.1007/s11947-009-0261-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-009-0261-4