Abstract
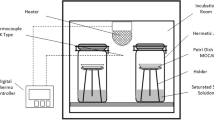
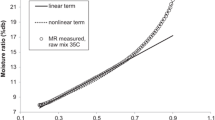
The desorption isotherms of chestnut flour and chestnut starch were determined at different temperatures (20°C, 35°C, 50°C, and 65°C) using gravimetric method. Desorption isotherms of potato starch were also determined in order to establish a comparison against desorption isotherms of chestnut starch. Several saturated salt solutions were selected to generate different water activities in the range of 0.09 to 0.91. Obtained desorption isotherms were of type II, according to Brunauer’s classification. Three-parameter Brunauer–Emmett–Teller and Guggenheim–Anderson–de Boer (GAB) models satisfactorily fitted the experimental data for all systems studies, although the last one can be considered better based on obtained statistical parameters and because it is applicable in a broader water activity range. The average monolayer moisture content (kilograms per kilogram d.b.) calculated by GAB model was 0.059 ± 0.007 for chestnut flour, 0.103 ± 0.021 for chestnut starch, and 0.060 ± 0.028 for potato starch. The net isosteric sorption heat, calculated by means of Clausius–Clapeyron equation, decreased when moisture content increased. The maximum values of net isosteric sorption heat (kilojoules per mole) were approximately 27.5 for chestnut flour, 16.0 for chestnut starch, and 33.0 for potato starch in the range of temperature from 20°C to 50°C.




Similar content being viewed by others
Abbreviations
- a w :
-
Water activity
- C :
-
GAB (Eq. 2) parameter
- C B :
-
BET (Eq. 1) parameter
- E :
-
Mean relative percentage deviation modulus
- E RMS :
-
Root mean square error (kg (kg d.b.)−1)
- H :
-
Heat of GAB model (kJ mol−1)
- h :
-
Sorption heat (kJ mol−1)
- h L :
-
Heat of water condensation (kJ mol−1)
- K :
-
GAB (Eq. 2) parameter
- n :
-
Number of samples
- N :
-
BET (Eq. 1) parameter
- R :
-
Ideal gas constant (8.314 J (mol K)−1)
- R 2 :
-
Coefficient of determination
- T :
-
Absolute temperature (K)
- X :
-
Equilibrium moisture content (kg (kg d.b.)−1)
- cal:
-
Calculated
- e:
-
Net isosteric
- exp:
-
Experimental
- M:
-
Monolayer of GAB model
- MB:
-
Monolayer of BET model
- N:
-
Multilayer
- o:
-
Pre-exponential
References
Aboubakar, N. Y. N., Scher, J., & Mbofung, C. M. F. (2008). Physicochemical, thermal properties and microstructure of six varieties of taro (Colocasia esculenta L. Schott) flours and starches. Journal of Food Engineering, 86, 294–305.
Aguilera, J. M., & Stanley, D. W. (1999). Microstructural principles of food processing and engineering (pp. 373–411). Maryland: Aspen.
Alexander, R. J. (1996). New starches for food application. Cereal food world, 41, 796–798.
Al-Muhtaseb, A. H., McMinn, W. A. M., & Magee, T. R. A. (2004a). Water sorption isotherms of starch powders. Part 1: mathematical description of experimental data. Journal of Food Engineering, 61, 297–307.
Al-Muhtaseb, A. H., McMinn, W. A. M., & Magee, T. R. A. (2004b). Water sorption isotherms of starch powders. Part 2: Thermodynamic characteristics. Journal of Food Engineering, 62, 135–142.
Ando, H., Tang, H., Watanabe, K., & Mitsunaga, T. (2002). Some physicochemical properties of large, medium and small granule starches in fractions of wheat grain. Food Science and Technology Research, 8, 24–27.
AOAC. (1995). Official methods of analysis. Washington, DC: Association of Official Analytical Chemistry.
Bellagha, S., Sahli, A., & Farhat, A. (2008). Desorption isotherms and isosteric heat of three Tunisian date cultivars. Food and Bioprocess Technology, 1, 270–275.
Brunauer, S., Emmet, P. H., & Teller, E. (1938). Adsorption of gases in multimolecular layers. Journal of the American Chemical Society, 60, 309–319.
Brunauer, S., Deming, L. S., Deming, W. E., & Teller, E. (1940). On a theory of the Van der Waals adsorption of gases. Journal of the American Chemical Society, 62, 1723–1732.
Demiate, I. M., Oetterer, M., & Wosiacki, G. (2001). Characterization of chestnut (Castanea sativa, Mill) starch for industrial utilization. Brazilian Archives of Biology and Technology, 44(1), 69–78.
Douzas, J. P., Marechal, P. A., Coquille, J. C., & Gervais, P. (1996). Microscopic study of starch gelatinization under high hydrostatic pressure. Journal of Agriculture and Food Chemistry, 44, 1403–1408.
Durakova, A. G., & Menkov, N. D. (2004). Moisture sorption characteristics of rice flour. Nahrung/Food, 48, 137–140.
FAO. (2009). <http://www.fao.org/infoods/directory_en.stm>
Fasina, O. O. (2006). Thermodynamic properties of sweet potato. Journal of Food Engineering, 75, 149–155.
Fortes, M., & Okos, M. R. (1980). Drying theories: Their bases and limitations as applied to food and grains. In A. S. Mujumdar (Ed.), Advances in drying, Vol 1 (pp. 119–154). New York: Hemisphere.
Greenspan, L. (1977). Humidity fixed points of binary saturated aqueous solutions. Journal of Research of the National Bureau of Standards Section A, 81, 89–102.
Jonquieres, A., & Fane, A. (1998). Modified BET models for modelling water vapour sorption in hydrophilic glassy polymers and systems deviating strongly from ideality. Journal of Applied Polymer Science, 67, 1415–1430.
Kim, S. S., & Bhowmik, S. R. (1994). Moisture sorption isotherms of concentrated yogurt and microwave vacuum dried yogurt powder. Journal of Food Engineering, 21, 157–175.
Leung, H. K. (1983). Water activity and other colligative properties of foods. In: ASAE annual meeting, paper no. 83-6508. Chicago, USA.
Lomauro, C. J., Bakshi, A. S., & Labuza, T. P. (1985). Evaluation of food moisture sorption isotherm equations. Part I. Fruit, vegetables and meat products. Lebensmittel Wissenschaft Technologie, 18, 111–115.
Mataix, J., Mañas, M., & Llopis, J. (1998). Tabla de composición de alimentos españoles. Granada: University of Granada.
McMinn, W. A. M., & Magee, T. R. A. (2003). Thermodynamic properties of moisture sorption of potato. Journal of Food Engineering, 60, 155–157.
McMinn, W. A. M., Al-Muhtaseb, A. H., & Magee, T. R. A. (2004). Moisture sorption characteristics of starch gels. Part II: Thermodynamic properties. Journal of Food Process Engineering, 27, 213–227.
McMinn, W. A. M., Al-Muhtaseb, A. H., & Magee, T. R. A. (2005). Enthalpy–entropy compensation in sorption phenomena of starch materials. Food Research International, 38, 505–510.
Rebar, V., Fischbach, E. R., Apostolopoulos, D., & Kokini, J. L. (1984). Thermodynamics of water and ethanol adsorption on four starches as model biomass separation systems. Biotechnology and Bioengineering, 26(5), 513–517.
Scott, W. J. (1957). Water relations of foods spoilage microorganisms. Advances in Food Research, 7, 83–127.
Scott, J. M., Hugh, J. C., & Colin, J. R. (1998). A simple and rapid colorimetric method for the determination of amylose in starch products. Starch, 50, 158–163.
Timmermann, E. O., Chirife, J., & Iglesias, H. A. (2001). Water sorption isotherms of foods and foodstuffs: BET or GAB parameters? Journal of Food Engineering, 48, 19–31.
Urquhart, A. R. (1959). Differential enthalpy of sorption in dried fruits. Journal of Food Engineering, 14, 327–335.
Van den Berg, C., & Bruin, S. (1981). Water activity and its estimation in food systems; theoretical aspects. In L. B. Rockland & C. F. Stewart (Eds.), Water activity: influences on food quality (pp. 1–61). New York: Academic.
Vázquez, G., Chenlo, F., & Moreira, R. (2001). Modeling of desorption isotherms of chestnut: Influence of temperature and evaluation of isosteric heats. Drying Technology, 19(6), 1189–1199.
Weast, R. C. (1982). Handbook of chemistry and physics. Boca Raton: CRC.
Yanniotis, S., Nikoletopoulos, P., Pappas, G. (1989). Sorption models for dried fruits. In: Proceedings of the 5th International Congress on Engineering and Food, Karlsruhe, Germany.
Acknowledgments
The authors acknowledge the financial support from the Ministerio de Educación y Ciencia of Spain and FEDER (CTQ 2007-62009/PPQ).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chenlo, F., Moreira, R., Prieto, D.M. et al. Desorption Isotherms and Net Isosteric Heat of Chestnut Flour and Starch. Food Bioprocess Technol 4, 1497–1504 (2011). https://doi.org/10.1007/s11947-009-0239-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-009-0239-2