Abstract
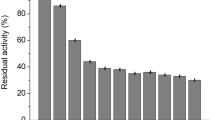
This research assesses the bench-scale application of a non-conventional support, bone particles, for glucoamylase (GA) immobilization and its subsequent use in cassava starch hydrolysis. Upon determining the appropriate conditions to immobilize GA onto chicken bone particles, such as pH, ionic strength, particle size, and enzyme load, bench-scale immobilization of commercial GA without further purification was performed. Under the selected conditions, 270 GA units per gram of support were adsorbed. Optimal temperature and thermal stability of immobilized GA were only slightly different from those of the free enzyme, while optimal pH became more acidic by about one unit. The feasibility of the use of this immobilized biocatalyst for high glucose syrup production from liquefied cassava starch, at bench scale in batch process using a stirred-tank reactor, was demonstrated. Repeated use of the GA-bone derivative showed that similar conversions to those achieved with soluble enzyme (dextrose equivalent = 98) were reached until the third batch and over 90% until the 25th batch.






Similar content being viewed by others
References
Adamczyk, Z., Jaszczólt, K., Siwek, B., & Weronski, P. (2004). Irreversible adsorption of particles at random-site surfaces. Journal of Chemical Physics, 120(23), 11155–11162. doi:10.1063/1.1712967.
Adlercreutz, P. (1993). Immobilized enzymes. In T. Nagodawithana, & G. Reed (Eds.), Enzymes in food processing (pp. 103–119, 3rd ed.). New York, USA: Academic.
American Water Works Association (1991). AWWA standard for granular activated carbon p. 11. Denver, USA: American Water Works Association.
Arica, M. Y., Alaeddinoğlu, N. G., & Hasirci, V. (1998). Immobilization of glucoamylase onto activated phema/egdma microspheres: Properties and application to a packed-bed reactor. Enzyme and Microbial Technology, 22(3), 152–157. doi:10.1016/S0141-0229(97)00139-7.
Atia, K. S., Ismail, S. A., & Dessouki, A. M. (2003). Immobilization of β-amylase using polyacrylamide polymer derivatives. Journal of Chemical Technology & Biotechnology, 78(8), 891–898. doi:10.1002/jctb.875.
Atkinson, B., Mavituna, F. (Eds.) (1991). Biochemical engineering and biotechnology handbook: Enzymes (2nd edn., pp. 521–605). Mexico: Stockton.
Bahar, T., & Çelebi, S. S. (1998). Characterization of glucoamylase immobilized on magnetic poly(styrene) particles. Enzyme and Microbial Technology, 23(5), 301–304. doi:10.1016/S0141-0229(98)00048-9.
Bahar, T., & Çelebi, S. S. (2000). Performance of immobilized glucoamylase in a magnetically stabilized fluidized bed reactor (MSFBR). Enzyme and Microbial Technology, 26(1), 28–33. doi:10.1016/S0141-0229(99)00129-5.
Bernfeld, P. (1955). Amylases, alpha and beta. In S. P. Colowick, & N. O. Kaplan (Eds.), Methods in enzymology, vol. 1 (pp. 149–158). USA: New York.
Bhargava, S., Frisner, H., & Bisgard-Frantzen, H. (2005). A process of producing a fermentation product. United States patent WO2005/113785 A2 (in English).
Brena, B. M. (1996). Reversible immobilization of enzymes using agarose-bound group-specific ligands. Ph. D. thesis. Faculty of Science and Technology, Uppsala University, Uppsala, Sweden. pp. 8.
Cabral, J. M. S., & Kennedy, J. F. (1991). Covalent and coordination immobilization of proteins. In R. F. Taylor (Ed.), Protein immobilization fundamentals and applications (pp. 73–138). USA: New York.
Carpio, C., González, P., Ruales, J., & Batista-Viera, F. (2000). Bone-bound enzymes for food industry application. Food Chemistry, 68(4), 403–409. doi:10.1016/S0308-8146(99)00193-4.
Chase, H. A. (1984). Prediction of the performance of preparative affinity chromatography. Journal of Chromatography A, 297, 179–202. doi:10.1016/S0021-9673(01)89041-5.
Corbishley, D. A., & Miller, W. (1984). Tapioca, arrowroot, and sago starches production. In R. L. Whistler, J. N. BeMiller, & E. F. Paschall (Eds.), Starch: Chemistry and technology (pp. 469–478, 2nd ed.). Orlando, USA: Academic.
Emnéus, J., & Gorton, L. (1993). Comparison between different inorganic supports for the immobilizaton of amyloglucosidase and α-amylase to be used in enzyme reactors in flow-injection systems. Analytica Chimica Acta, 276(2), 303–318. doi:10.1016/0003-2670(93)80398-5.
Fang, F., & Szleifer, I. (2001). Kinetics and thermodynamics of protein adsorption: A generalized molecular theoretical approach. Biophysical Journal, 80, 2568–2589.
Findlay, C. J., Parkin, K. L., & Yada, R. Y. (1986). Bone as a solid support for the immobilization of enzymes. Biotechnology Letters, 8(9), 649–652. doi:10.1007/BF01025975.
Flynn, A., & Johnson, D. B. (1978). Some factors affecting the stability of glucoamylase immobilized on hornblende and other inorganic supports. Biotechnology and Bioengineering, 20(9), 1445–1454. doi:10.1002/bit.260200909.
Howling, D. (1992). Glucose syrup: Production, properties and applications. In F. W. Schenck, & R. E. Hebeda (Eds.), Starch hydrolysis products (pp. 277–317). New York, USA: VCH.
Kennedy, J. F., & Cabral, J. M. S. (1987). Enzyme immobilization. In H. J. Rehm, & G. Reed (Eds.), Biotechnology, vol. 72 (pp. 347–404). Weinheim: VCH Verlagsgesellschaft.
Kierstan, M. P. J., & Coughlan, M. P. (1991). Immobilization of protein by noncovalent procedures: Principles and applications. In R. F. Taylor (Ed.), Protein immobilization, fundamentals and applications (pp. 13–71). New York, USA: Marcel Dekker.
Klibanov, A. M., & Ahern, T. J. (1987). Thermal stability of proteins. In D. L. Oxender, & C. F. Fox (Eds.), Protein engineering (pp. 213–218). New York, USA: Liss.
Kovalenko, G. A., & Perminova, L. V. (2008). Immobilization of glucoamylase by adsorption on carbon supports and its application for heterogeneous hydrolysis of dextrin. Carbohydrate Research, 343, 1202–1211. doi:10.1016/j.carres.2008.02.006.
Kovalenko, G. A., Komova, O. V., Simakov, A. V., Khomov, V. V., & Rudina, N. A. (2002). Macrostructured carbonized ceramics as adsorbents for immobilization of glucoamylase. Journal of Molecular Catalysis. A, Chemical, 182–183, 73–80. doi:10.1016/S1381-1169(01)00479-4.
Kovalenko, G. A., Perminova, L. V., Plaksin, G. V., Chuenko, T. V., Komova, O. V., & Rudina, N. A. (2006). Immobilized glucoamylase: A biocatalyst of dextrin hydrolysis. Applied Biochemistry and Microbiology, 42(2), 145–149. doi:10.1134/S0003683806020050.
Kovalenko, G. A., Perminova, L. V., Terent’eva, T. G., & Plaksin, G. V. (2007). Catalytic properties of glucoamylase immobilized on synthetic carbon material sibunit. Applied Biochemistry and Microbiology, 43(4), 374–378. doi:10.1134/S0003683807040023.
Lee, D. D., Lee, Y. Y., Reilly, P., Collins, E. J., & Tsao, G. T. (1976). Pilot plant production of glucose with glucoamylase immobilized to porous silica. Biotechnology and Bioengineering, 18(2), 253–267. doi:10.1002/bit.260180210.
Miller, E. (1998). Immobilization of glucoamylase on polyamide nets. Acta Biotechnologica, 18(2), 135–146. doi:10.1002/abio.370180208.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31(3), 426–428. doi:10.1021/ac60147a030.
Montgomery, D. C. (2001). Design and analysis of experiments pp. 21–54, 427–510. New York, USA: Wiley.
Mukataka, S., Negishi, S., Sato, S., & Takahashi, J. (1993). Effect of substrate presoaking treatment of support materials on the activity of immobilized glucoamylase. Enzyme and Microbial Technology, 15(3), 229–233. doi:10.1016/0141-0229(93)90142-O.
Mulvihill, P. J. (1992). Crystalline and liquid dextrose products: Production, properties and applications. In F. W. Schenck, & R. E. Hebeda (Eds.), Starch hydrolysis products worldwide technology, production, and applications (pp. 121–176). New York, USA: VCH.
Negishi, S., Sato, S., Mukataka, S., & Takahashi, J. (1989). Utilization of powdered pig bone as a support for immobilization of lipase. Journal of Fermentation and Bioengineering, 67(5), 350–355. doi:10.1016/0922-338X(89)90254-7.
Nolsøe, H., & Undeland, I. (2008). The acid and alkaline solubilization process for the isolation of muscle proteins: State of the art. Food Bioprocess Technol. doi:10.1007/s11947-008-0088-4
Polakovič, M., & Bryjak, J. (2002). Modelling of the kinetics of thermal inactivation of glucoamylase from Aspergillus niger. Journal of Molecular Catalysis. B, Enzymatic, 19–20, 443–450. doi:10.1016/S1381-1177(02)00197-2.
Rani, A. S., Das, M. L. M., & Satyanarayana, S. (2000). Preparation and characterization of amyloglucosidase adsorbed on activated charcoal. Journal of Molecular Catalysis. B, Enzymatic, 10(5), 471–476. doi:10.1016/S1381-1177(99)00116-2.
Reeve, A. (1992). Starch hydrolysis: Processes and equipment. In F. W. Shenck, & R. E. Hebeda (Eds.), Starch hydrolysis products worldwide technology, production, and applications (pp. 79–120). New York, USA: VCH.
Roig, M. G., Slade, A., Kennedy, J. F ., Taylor, D. W., & Garaita, M. G. (1995). Investigations of stabilities, ph, and temperature profiles and kinetic parameters of glucoamylase immobilized on plastic supports. Applied Biochemistry and Biotechnology, 50(1), 11–33. doi:10.1007/BF02788037.
Rudra, S. G., Shivhare, U. S., Basu, S., & Sarkar, B. C. (2008). Thermal inactivation kinetics of peroxidase in coriander leaves. Food Bioprocess Technol, 1(2), 187–195. doi:10.1007/s11947-007-0013-2.
Rugh, S., Nielsen, T., & Poulsen, P. B. (1979). Application possibilities of a novel immobilized glucoamylase. Starch/Stärke, 31(10), S 333–337.
Sadana, A., & Henley, J. P. (1987). Single-step unimolecular non-first-order enzyme deactivation kinetics. Biotechnology and Bioengineering, 30(6), 717–723. doi:10.1002/bit.260300604.
Sasvári, Z., & Asbóth, B. (1999). Crosslinking of glucoamylases via carbohydrates hardly affects catalysis but impairs stability. Biotechnology and Bioengineering, 63(4), 459–463. doi:10.1002/(SICI)1097-0290(19990520)63:4<459::AID-BIT9>3.0.CO;2-I.
Schafhauser, D. Y., & Storey, K. B. (1992a). Coimmobilized amyloglucosidase, pululanase and glucose isomerase on biobonetm. Applied Biochemistry and Biotechnology, 36, 63–74. doi:10.1007/BF02950775.
Schafhauser, D. Y., & Storey, K. B. (1992b). Immobilization of amyloglucosidase onto granular chicken bone. Applied Biochemistry and Biotechnology, 32(2), 89–109. doi:10.1007/BF02922151.
Schenck, F. W., & Hebeda, R. E. (1992). Starch hydrolysis products: An introduction and history. In F. W. Schenck, & R. E. Hebeda (Eds.), Starch hydrolysis products worldwide technology production and applications (pp. 1–21). New York, USA: VCH.
Silva, R. N., Asquieri, E. R., & Fernandes, K. F. (2005). Immobilization of Aspergillus niger glucoamylase onto a polyaniline polymer. Process Biochemistry, 40(3), 1155–1159. doi:10.1016/j.procbio.2004.04.006.
Soriano, R., Bautista, L. F., Martínez, M., & Aracil, J. (1999). Adsorption isotherms of the Aspergillus niger glucoamylases i and ii on the anionic exchanger deae-toyopearl 650+. Journal of Chemical Technology & Biotechnology, 74(3), 199–203. doi:10.1002/(SICI)1097-4660(199903)74:3<199::AID-JCTB42>3.0.CO;2-B.
Swinkels, J. J. (1985). Sources of starch, its chemistry and physics. In G. M. A. Van Beynum, & J. A. Roels (Eds.), Starch conversion technology (pp. 15–46). New York, USA: Marcel Dekker.
Tanriseven, A., Uludağ, Y. B., & Doğan, Ş. (2002). A novel method for the immobilization of glucoamylase to produce glucose from maltodextrin. Enzyme and Microbial Technology, 30(3), 406–409. doi:10.1016/S0141-0229(02)00004-2.
Torres, R., Pessela, B. C. C., Mateo, C., Ortiz, C., Fuentes, M., Guisán, J. M., & Fernandes-Lafuente, R. (2004). Reversible immobilization of glucoamylase by ionic adsorption on sepabeads coated with polyethylenimine. Biotechnology Progress, 20(4), 1297–300. doi:10.1021/bp049943g.
Trevan, M. D. (1980). Immobilized enzymes. An introduction and applications in biotechnology p. 20. New York, USA: Wiley.
Trinder, P. (1969). Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Annals of Clinical Biochemistry, 6(1), 24–28.
Varavinit, S., Chaokasem, N., & Shobsngob, S. (2001). Covalent immobilization of a glucoamylase to bagase dialdehyde cellulose. World Journal of Microbiology & Biotechnology, 17(7), 721–725. doi:10.1023/A:1012984802624.
Velayudhan, A., & Horváth, C. (1994). Adsorption and ion-exchange isotherms in preparative chromatography. Journal of Chromatography A, 663(1), 1–10. doi:10.1016/0021-9673(94)80490-7.
Waliszweski, K. N., Garcia-Alvarado, M., & Medina, J. C. (1992). Kinetics of enzyme hydrolysis of cassava flour starch-optimization and modelling. International Journal of Food Science & Technology, 27(4), 465–472.
Woodward, J. (1985). Immobilised enzymes: Adsorption and covalent coupling. In J. Woodward (Ed.),Immobilised cells and enzymes: A practical approach (pp. 3,13–15). Washington, DC, USA: IRL.
Acknowledgments
This research was supported by the International Program in the Chemical Sciences (IPICS), Uppsala University, Sweden (project ECU-01). The support of LATSOBIO and LANFOOD-IPICS networks is specially acknowledged. The authors thank to Engrs. N. Espín, O. Proaño and P. Polit from Escuela Politécnica Nacional for their valuable contribution to the development of experimental work. Prof. Dr. Jan D. Miller, Chair Department of Metallurgy, College of Mines and Earth Science, University of Utah and Dr. Ximena Díaz are kindly acknowledged for the surface area analysis of the supports.
Author information
Authors and Affiliations
Corresponding author
Additional information
Grant: International Program in the Chemical Sciences (IPICS), Uppsala University, Sweden (project ECU-01).
Rights and permissions
About this article
Cite this article
Carpio, C., Escobar, F., Batista-Viera, F. et al. Bone-Bound Glucoamylase as a Biocatalyst in Bench-Scale Production of Glucose Syrups from Liquefied Cassava Starch. Food Bioprocess Technol 4, 566–577 (2011). https://doi.org/10.1007/s11947-008-0164-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-008-0164-9