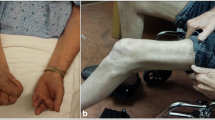
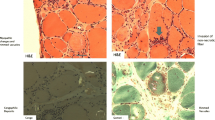
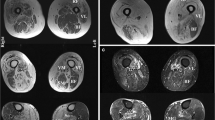
Abstract
Purpose of the review
The purpose of this paper is to review the recent findings that pertain to the diagnosis and treatment of sporadic inclusion body myositis.
Recent findings
New evidence highlighting lack of correlation between the presence of cytosolic 5′-nucleotidase 1A antibody with any of the clinical features, or laboratory findings in inclusion body myositis. New studies emphasizing heterogeneity and showing separation of inclusion body myositis patients into separate trajectories. The failure of bimagrumab and arimoclomol as potential treatments of inclusion body myositis and the mixed results of the sirolimus trial in sporadic inclusion body myositis.
Summary
A significant gap exists in understanding the heterogeneity of sporadic inclusion body myositis. Recent evidence suggests that cytosolic 5′-nucleotidase 1A antibody does not provide significant clinical or laboratory differentiation between antibody-positive and negative patients. Despite recent failures in the clinical trials of arimoclomol and bimagrumab, sirolimus showed mixed results, and a larger definitive trial is needed.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Keshishian A, Greenberg SA, Agashivala N, Baser O, Johnson K. Health care costs and comorbidities for patients with inclusion body myositis. Curr Med Res Opin. 2018;34:1679–85.
Callan A, Capkun G, Vasanthaprasad V, Freitas R, Needham M. A systematic review and meta-analysis of prevalence studies of sporadic inclusion body myositis. J Neuromuscul Dis. 2017;4(2):127–37. https://doi.org/10.3233/JND-160198.
Dobloug GC, Antal EA, Sveberg L, Garen T, Bitter H, Stjärne J, et al. High prevalence of inclusion body myositis in Norway; a population-based clinical epidemiology study. Eur J Neurol. 2015;22(4):672–e41. https://doi.org/10.1111/ene.12627 Epub 2014 Dec 21.
Needham M, James I, Corbett A, Day T, Christiansen F, Phillips B, et al. Sporadic inclusion body myositis: phenotypic variability and influence of HLA- DR3 in a cohort of 57 Australian cases. J Neurol Neurosurg Psychiatry. 2008;79:1056–60.
Cox FM, Verschuuren JJ, Verbist BM, Niks EH, Wintzen AR, Badrising UA. Detecting dysphagia in inclusion body myositis. J Neurol. 2009;256(12):2009–13. https://doi.org/10.1007/s00415-009-5229-9.
Lin AY, Clapp M, Karanja E, Dooley K, Weihl CC, Wang LH. A cross-sectional study of hand function in inclusion body myositis: implications for functional rating scale. Neuromuscul Disord. 2019;S0960-8966(19):31229–5. https://doi.org/10.1016/j.nmd.2019.12.002.
• Nicolau S, Liewluck T, Milone M. Myopathies with finger flexor weakness: not only inclusion-body myositis. Muscle Nerve. 2020;62(4):445–54. https://doi.org/10.1002/mus.26914. An important overview of the differential diagnosis of myopathies that can cause finger flexor weakness.
Pluk H, van Hoeve BJ, van Dooren SH, Stammen-Vogelzangs J, van der Heijden A, Schelhaas HJ, et al. Autoantibodies to cytosolic 5'-nucleotidase 1A in inclusion body myositis. Ann Neurol. 2013;73(3):397–407. https://doi.org/10.1002/ana.23822.
Larman HB, Salajegheh M, Nazareno R, Lam T, Sauld J, Steen H, et al. Cytosolic 5'-nucleotidase 1A autoimmunity in sporadic inclusion body myositis. Ann Neurol. 2013;73(3):408–18. https://doi.org/10.1002/ana.23840.
Herbert MK, Stammen-Vogelzangs J, Verbeek MM, Rietveld A, Lundberg IE, Chinoy H, et al. Disease specificity of autoantibodies to cytosolic 5'-nucleotidase 1A in sporadic inclusion body myositis versus known autoimmune diseases. Ann Rheum Dis. 2016;75(4):696–701. https://doi.org/10.1136/annrheumdis-2014-206691.
Liewluck T. Anti-cytosolic 5'-nucleotidase 1A (cN1A) autoantibodies in motor neuron diseases. Neurology. 2017 Nov 7;89(19):2017–8. https://doi.org/10.1212/WNL.0000000000004610.
Lloyd TE, Christopher-Stine L, Pinal-Fernandez I, Tiniakou E, Petri M, Baer A, et al. Cytosolic 5′-nucleotidase 1A as a target of circulating autoantibodies in autoimmune diseases. Arthritis Care Res. 2016;68:66–71.
Lilleker JB, Rietveld A, Pye SR, Mariampillai K, Benveniste O, Peeters MTJ, et al. Cytosolic 5′-nucleotidase 1A autoantibody profile and clinical characteristics in inclusion body myositis. Ann Rheum Dis. 2017;76:862–8.
Goyal NA, Cash TM, Alam U, Enam S, Tierney P, Araujo N, et al. Seropositivity for NT5c1A antibody in sporadic inclusion body myositis predicts more severe motor, bulbar and respiratory involvement. J Neurol Neurosurg Psychiatry. 2016;87:373–8.
•• Paul P, Liewluck T, Ernste FC, Mandrekar J, Milone M. Anti-cN1A antibodies do not correlate with specific clinical, electromyographic, or pathological findings in sporadic inclusion body myositis. Muscle Nerve. 2020;29. https://doi.org/10.1002/mus.27157. A single center study showing no correlation between anti-cN1A antibody and the clinical, laboratory, or pathologic findings in sporadic inclusion body myositis.
•• Ikenaga C, Findlay AR, Goyal NA, Robinson S, Cauchi J, Hussain Y, et al. Clinical utility of anti-cytosolic 5'-nucleotidase 1A antibody in idiopathic inflammatory myopathies. Ann Clin Transl Neurol. 2021;8(3):571–8. https://doi.org/10.1002/acn3.51294. A five center larger study showing again the lack of correlation of anti-cN1A antibody with clinical and pathologic features of sporadic inclusion body myositis and seropositivity in other myopathies.
Oldroyd AGS, Lilleker JB, Williams J, Chinoy H, Miller JAL. Long-term strength and functional status in inclusion body myositis and identification of trajectory subgroups. Muscle Nerve. 2020;62(1):76–82. https://doi.org/10.1002/mus.26859.
Cortese A, Machado P, Morrow J, Dewar L, Hiscock A, Miller A, et al. Longitudinal observational study of sporadic inclusion body myositis: implications for clinical trials. Neuromuscul Disord. 2013;23(5):404–12. https://doi.org/10.1016/j.nmd.2013.02.010.
Tasca G, Monforte M, De Fino C, Kley RA, Ricci E, Mirabella M. Magnetic resonance imaging pattern recognition in sporadic inclusion-body myositis. Muscle Nerve. 2015;52(6):956–62. https://doi.org/10.1002/mus.24661.
Ansari B, Salort-Campana E, Ogier A, Le Troter PDA, De Sainte MB, Guye M, et al. Quantitative muscle MRI study of patients with sporadic inclusion body myositis. Muscle Nerve. 2020;61(4):496–503. https://doi.org/10.1002/mus.26813.
Noto Y, Shiga K, Tsuji Y, Kondo M, Tokuda T, Mizuno T, et al. Contrasting echogenicity in flexor digitorum profundus-flexor carpi ulnaris: a diagnostic ultrasound pattern in sporadic inclusion body myositis. Muscle Nerve. 2014;49(5):745–8. https://doi.org/10.1002/mus.24056.
Albayda J, Christopher-Stine L, Bingham Iii CO, Paik JJ, Tiniakou E, Billings S, et al. Pattern of muscle involvement in inclusion body myositis: a sonographic study. Clin Exp Rheumatol. 2018;36(6):996–1002.
Karvelas KR, Xiao T, Langefeld CD, Walker FO, Pathak S, Caress JB, et al. Assessing the accuracy of neuromuscular ultrasound for inclusion body myositis. Muscle Nerve. 2019;59(4):478–81. https://doi.org/10.1002/mus.26411.
Rose MR, ENMC IBM. Working Group. 188th ENMC International Workshop: Inclusion Body Myositis, 2-4 December 2011, Naarden, The Netherlands. Neuromuscul Disord. 2013;23(12):1044–55. https://doi.org/10.1016/j.nmd.2013.08.007.
Lloyd T. E., Mammen AI, Amato AA, Weiss MD, Needham M, Greenberg SA. Evaluation and construction of diagnostic criteria for inclusion body myositis. Neurology. 2014;83:426–33.
Felice KJ, North WA. Inclusion body myositis in Connecticut: observations in 35 patients during an 8-year period. Medicine (Baltimore). 2001;80(5):320–7. https://doi.org/10.1097/00005792-200109000-00006.
Abuzinadah AR, Joseph JT, Korngut L. Amyloid myoneuropathy mimicking inclusion body myositis. Can J Neurol Sci. 2013;40(2):255–8.
Heim A, Hamasaki A, Dimachkie M, Pasnoor M, Jawdat O, Statland J, et al. Amyloid myopathy as an inclusion body myositis mimic. RRNMF Neuromuscular Journal. 2020;1(4):28–32.
Greenberg SA. Inclusion body myositis: clinical features and pathogenesis. Nat Rev Rheumatol. 2019;15:257–72.
Alexanderson H. Exercise in inflammatory myopathies, including inclusion body myositis. Curr Rheumatol Rep. 2012;14(3):244–51. https://doi.org/10.1007/s11926-012-0248-4.
Oh TH, Brumfield KA, Hoskin TL, Kasperbauer JL, Basford JR. Dysphagia in inclusion body myositis: clinical features, management, and clinical outcome. Am J Phys Med Rehabil. 2008;87(11):883–9. https://doi.org/10.1097/PHM.0b013e31818a50e2.
Smith RC, Lin BK. Myostatin inhibitors as therapies for muscle wasting associated with cancer and other disorders. Curr Opin Support Palliat Care. 2013;7(4):352–60. https://doi.org/10.1097/SPC.0000000000000013.
•• Hanna MG, Badrising UA, Benveniste O, Lloyd TE, Needham M, Chinoy H, et al. RESILIENT Study Group. Safety and efficacy of intravenous bimagrumab in inclusion body myositis (RESILIENT): a randomised, double-blind, placebo-controlled phase 2b trial. Lancet Neurol. 2019;18(9):834–44. https://doi.org/10.1016/S1474-4422(19)30200-5. An important clinical trial showing lack of effect of bimagrumab on the primary endpoint.
Ahmed M, Machado PM, Miller A, Spicer C, Herbelin L, He J, et al. Targeting protein homeostasis in sporadic inclusion body myositis. Sci Transl Med. 2016;8(331):331ra41. https://doi.org/10.1126/scitranslmed.aad4583.
Orphazyme announces topline results from pivotal trial of arimoclomol for Inclusion Body Myositis (IBM). https://tools.eurolandir.com/tools/Pressreleases/GetPressRelease/?ID=3890677&lang=en-GB&companycode=dk-orpha&v= . Last accessed 4/9/2021
•• Benveniste O, Hogrel J-Y, Belin L, Annoussamy M, Bachasson D, Rigolet A, et al. Sirolimus for treatment of patients with inclusion body myositis: a randomised, double-blind, placebo-controlled, proof-of-concept, phase 2b trial. Lancet Rheumatol. 2020. https://doi.org/10.1016/S2665-9913(20)30280-0. An important clinical trial showing a beneficial signal of sirolimus on several secondary endpoints necessitating further exploration in a definitive phase 3 trial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Kushlaf has served as a consultant on advisory boards for Alexion Pharmaceuticals, Catalyst Pharmaceuticals, Sanofi Genzyme, PTC therapeutics, and Takeda. Dr. Kushlaf serves on the speaker bureaus of Akcea, Catalyst Pharmaceuticals, and Sanofi Genzyme. None of these disclosures was relevant to this editorial.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neuromuscular Disorders
Rights and permissions
About this article
Cite this article
Kushlaf, H. Update on the Diagnostic and Therapeutic Landscape of Sporadic Inclusion Body Myositis. Curr Treat Options Neurol 23, 27 (2021). https://doi.org/10.1007/s11940-021-00681-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s11940-021-00681-5