Abstract
Purpose of review
Congenital myasthenia syndromes are clinically and genetically heterogeneous but treatable conditions. Careful selection of drug therapy is paramount as the same drug can be effective, ineffective, and even harmful in different congenital myasthenia syndromes. The purpose of this article is to review current treatment options for these conditions.
Recent findings
Next-generation sequencing has accelerated the discovery of new genes and facilitated the description of novel congenital myasthenic syndromes. Retrospective therapy data from these newly identified syndromes has provided additional insight on the management of these conditions. Cholinergic agents, β-adrenergic agonists, and open-channel blockers remain the principal treatment modalities, and their optimal use depends on an accurate genetic diagnosis and the timely clinical recognition of the disease. In particular, pyridostigmine, usually a first-line agent, should be avoided in DOK7, acetylcholinesterase deficiency, and slow-channel congenital myasthenic syndromes. Beta-adrenergic agonists have been recognized as a first-line agent for a number of congenital myasthenic syndromes, particularly DOK7 and acetylcholinesterase deficiency, whereas long-lived open-channel blockers of the acetylcholine receptor (AChR) ion channel are indicated for the slow-channel congenital myasthenic syndrome. Beta-adrenergic agonists additionally have an important adjunct treatment for congenital myasthenia syndrome due to glycosylation defects, fast channel syndrome, AChR deficiency, and choline acetyltransferase deficiency (ChaT) and therefore may be particularly important in the treatment of syndromes due to defects in motor endplate development and repair.
Summary
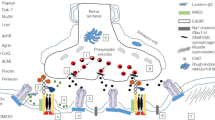
Unlike in autoimmune myasthenia gravis, there is no role for immunotherapy in congenital myasthenic syndromes. If available, a genetic diagnosis should drive the choice for a first-line treatment agent between cholinergic agents, β-adrenergic agents, and open-channel blockers. Evaluation and supportive care at centers with experience in these rare syndromes likely are paramount in achieving optimal outcomes. Furthermore, gene discovery for congenital myasthenic syndromes has provided novel insights on the role of protein glycosylation, endplate maintenance and repair, and synaptic vesicle exocytosis in neuromuscular transmission. These insights may lead to new therapeutic strategies in both congenital and autoimmune myasthenic diseases in the future.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
McMacken G, Abicht A, Evangelista T, Spendiff S, Lochmuller H. The increasing genetic and phenotypical diversity of congenital myasthenic syndromes. Neuropediatrics. 2017;48(4):294–308. https://doi.org/10.1055/s-0037-1602832.
Shieh PB, Oh SJ. Congenital myasthenic syndromes. Neurol Clin. 2018;36(2):367–78. https://doi.org/10.1016/j.ncl.2018.01.007.
Engel AG. The therapy of congenital myasthenic syndromes. Neurotherapeutics. 2007;4(2):252–7. https://doi.org/10.1016/j.nurt.2007.01.001.
•• Lee M, Beeson D, Palace J. Therapeutic strategies for congenital myasthenic syndromes. Ann N Y Acad Sci. 2018;1412(1):129–36. https://doi.org/10.1111/nyas.13538. Recent review of congenital myasthenic syndrome therapies that includes a highly informative illustrated algorithm for the treatment of main disease subtypes.
•• Engel AG, Shen XM, Selcen D, Sine SM. Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment. Lancet Neurol. 2015;14(5):461. https://doi.org/10.1016/S1474-4422(15)00010-1. Largest single-center series of congenital myasthenic syndrome patients with detailed information on pathogenesis, diagnosis, and treatment.
• Senderek J, Muller JS, Dusl M, Strom TM, Guergueltcheva V, Diepolder I, et al. Hexosamine biosynthetic pathway mutations cause neuromuscular transmission defect. Am J Hum Genet. 2011;88(2):162–72. https://doi.org/10.1016/j.ajhg.2011.01.008. Initial report of a congenital myasthenia syndrome linked to a glycosylation pathway defect.
Janas JS, Barohn RJ. A clinical approach to the congenital myasthenic syndromes. J Child Neurol. 1995;10(2):168–9. https://doi.org/10.1177/088307389501000221.
Abicht A, Müller JS, Lochmüller H. GeneReviews®: Congenital myasthenic syndromes. Seattle (WA): University of Washington, Seattle; 2003 May 9 [Updated 2016 Jul 14].
Verma S, Mazell SN, Shah DA. Amifampridine phosphate in congenital myasthenic syndrome. Muscle Nerve. 2016;54(4):809–10. https://doi.org/10.1002/mus.25230.
Sadeh M, Shen XM, Engel AG. Beneficial effect of albuterol in congenital myasthenic syndrome with epsilon-subunit mutations. Muscle Nerve. 2011;44(2):289–91. https://doi.org/10.1002/mus.22153.
Cossins J, Belaya K, Hicks D, Salih MA, Finlayson S, Carboni N, et al. Congenital myasthenic syndromes due to mutations in ALG2 and ALG14. Brain. 2013;136(Pt 3):944–56. https://doi.org/10.1093/brain/awt010.
Belaya K, Finlayson S, Slater CR, Cossins J, Liu WW, Maxwell S, et al. Mutations in DPAGT1 cause a limb-girdle congenital myasthenic syndrome with tubular aggregates. Am J Hum Genet. 2012;91(1):193–201. https://doi.org/10.1016/j.ajhg.2012.05.022.
Selcen D, Shen XM, Brengman J, Li Y, Stans AA, Wieben E, et al. DPAGT1 myasthenia and myopathy: genetic, phenotypic, and expression studies. Neurology. 2014;82(20):1822–30. https://doi.org/10.1212/wnl.0000000000000435.
Selcen D, Shen XM, Milone M, Brengman J, Ohno K, Deymeer F, et al. GFPT1-myasthenia: clinical, structural, and electrophysiologic heterogeneity. Neurology. 2013;81(4):370–8. https://doi.org/10.1212/WNL.0b013e31829c5e9c.
Rodriguez Cruz PM, Belaya K, Basiri K, Sedghi M, Farrugia ME, Holton JL, et al. Clinical features of the myasthenic syndrome arising from mutations in GMPPB. J Neurol Neurosurg Psychiatry. 2016;87(8):802–9. https://doi.org/10.1136/jnnp-2016-313163.
Engel AG, Selcen D, Shen XM, Milone M, Harper CM. Loss of MUNC13-1 function causes microcephaly, cortical hyperexcitability, and fatal myasthenia. Neurol Genet. 2016;2(5):e105. https://doi.org/10.1212/nxg.0000000000000105.
O'Connor E, Topf A, Muller JS, Cox D, Evangelista T, Colomer J, et al. Identification of mutations in the MYO9A gene in patients with congenital myasthenic syndrome. Brain. 2016;139(Pt 8):2143–53. https://doi.org/10.1093/brain/aww130.
Regal L, Shen XM, Selcen D, Verhille C, Meulemans S, Creemers JW, et al. PREPL deficiency with or without cystinuria causes a novel myasthenic syndrome. Neurology. 2014;82(14):1254–60. https://doi.org/10.1212/wnl.0000000000000295.
Burke G, Cossins J, Maxwell S, Owens G, Vincent A, Robb S, et al. Rapsyn mutations in hereditary myasthenia: distinct early- and late-onset phenotypes. Neurology. 2003;61(6):826–8.
Visser AC, Laughlin RS, Litchy WJ, Benarroch EE, Milone M. Rapsyn congenital myasthenic syndrome worsened by fluoxetine. Muscle Nerve. 2017;55(1):131–5. https://doi.org/10.1002/mus.25244.
Tsujino A, Maertens C, Ohno K, Shen XM, Fukuda T, Harper CM, et al. Myasthenic syndrome caused by mutation of the SCN4A sodium channel. Proc Natl Acad Sci U S A. 2003;100(12):7377–82. https://doi.org/10.1073/pnas.1230273100.
Bauche S, O'Regan S, Azuma Y, Laffargue F, McMacken G, Sternberg D, et al. Impaired presynaptic high-affinity choline transporter causes a congenital myasthenic syndrome with episodic apnea. Am J Hum Genet. 2016;99(3):753–61. https://doi.org/10.1016/j.ajhg.2016.06.033.
Shen XM, Selcen D, Brengman J, Engel AG. Mutant SNAP25B causes myasthenia, cortical hyperexcitability, ataxia, and intellectual disability. Neurology. 2014;83(24):2247–55. https://doi.org/10.1212/wnl.0000000000001079.
Shen XM, Scola RH, Lorenzoni PJ, Kay CS, Werneck LC, Brengman J, et al. Novel synaptobrevin-1 mutation causes fatal congenital myasthenic syndrome. Annals of Clinical and Translational Neurology. 2017;4(2):130–8. https://doi.org/10.1002/acn3.387.
Whittaker RG, Herrmann DN, Bansagi B, Hasan BA, Lofra RM, Logigian EL, et al. Electrophysiologic features of SYT2 mutations causing a treatable neuromuscular syndrome. Neurology. 2015;85(22):1964–71. https://doi.org/10.1212/wnl.0000000000002185.
O'Grady GL, Verschuuren C, Yuen M, Webster R, Menezes M, Fock JM, et al. Variants in SLC18A3, vesicular acetylcholine transporter, cause congenital myasthenic syndrome. Neurology. 2016;87(14):1442–8. https://doi.org/10.1212/wnl.0000000000003179.
Bestue-Cardiel M, Saenz de Cabezon-Alvarez A, Capablo-Liesa JL, Lopez-Pison J, Pena-Segura JL, Martin-Martinez J, et al. Congenital endplate acetylcholinesterase deficiency responsive to ephedrine. Neurology. 2005;65(1):144–6. https://doi.org/10.1212/01.wnl.0000167132.35865.31.
Liewluck T, Selcen D, Engel AG. Beneficial effects of albuterol in congenital endplate acetylcholinesterase deficiency and Dok-7 myasthenia. Muscle Nerve. 2011;44(5):789–94. https://doi.org/10.1002/mus.22176.
Huze C, Bauche S, Richard P, Chevessier F, Goillot E, Gaudon K, et al. Identification of an agrin mutation that causes congenital myasthenia and affects synapse function. Am J Hum Genet. 2009;85(2):155–67. https://doi.org/10.1016/j.ajhg.2009.06.015.
Selcen D, Milone M, Shen XM, Harper CM, Stans AA, Wieben ED, et al. Dok-7 myasthenia: phenotypic and molecular genetic studies in 16 patients. Ann Neurol. 2008;64(1):71–87. https://doi.org/10.1002/ana.21408.
Selcen D, Ohkawara B, Shen XM, McEvoy K, Ohno K, Engel AG. Impaired synaptic development, maintenance, and neuromuscular transmission in LRP4-related myasthenia. JAMA Neurology. 2015;72(8):889–96. https://doi.org/10.1001/jamaneurol.2015.0853.
Ohkawara B, Cabrera-Serrano M, Nakata T, Milone M, Asai N, Ito K, et al. LRP4 third beta-propeller domain mutations cause novel congenital myasthenia by compromising agrin-mediated MuSK signaling in a position-specific manner. Hum Mol Genet. 2014;23(7):1856–68. https://doi.org/10.1093/hmg/ddt578.
Gallenmuller C, Muller-Felber W, Dusl M, Stucka R, Guergueltcheva V, Blaschek A, et al. Salbutamol-responsive limb-girdle congenital myasthenic syndrome due to a novel missense mutation and heteroallelic deletion in MUSK. Neuromuscul Disord. 2014;24(1):31–5. https://doi.org/10.1016/j.nmd.2013.08.002.
Mihaylova V, Salih MA, Mukhtar MM, Abuzeid HA, El-Sadig SM, von der Hagen M, et al. Refinement of the clinical phenotype in musk-related congenital myasthenic syndromes. Neurology. 2009;73(22):1926–8. https://doi.org/10.1212/WNL.0b013e3181c3fce9.
Harper CM, Engel AG. Quinidine sulfate therapy for the slow-channel congenital myasthenic syndrome. Ann Neurol. 1998;43(4):480–4. https://doi.org/10.1002/ana.410430411.
Harper CM, Fukodome T, Engel AG. Treatment of slow-channel congenital myasthenic syndrome with fluoxetine. Neurology. 2003;60(10):1710–3.
Edgeworth H. A report of progress on the use of ephedrine in a case of myasthenia gravis. J Am Med Assoc. 1930;94(15):1. https://doi.org/10.1001/jama.1930.27120410003009c.
Rodriguez Cruz PM, Palace J, Ramjattan H, Jayawant S, Robb SA, Beeson D. Salbutamol and ephedrine in the treatment of severe AChR deficiency syndromes. Neurology. 2015;85(12):1043–7. https://doi.org/10.1212/wnl.0000000000001952.
Beeson D. Congenital myasthenic syndromes: recent advances. Curr Opin Neurol. 2016;29(5):565–71. https://doi.org/10.1097/wco.0000000000000370.
Ohno K, Wang HL, Milone M, Bren N, Brengman JM, Nakano S, et al. Congenital myasthenic syndrome caused by decreased agonist binding affinity due to a mutation in the acetylcholine receptor epsilon subunit. Neuron. 1996;17(1):157–70.
Milone M, Shen XM, Selcen D, Ohno K, Brengman J, Iannaccone ST, et al. Myasthenic syndrome due to defects in rapsyn: clinical and molecular findings in 39 patients. Neurology. 2009;73(3):228–35. https://doi.org/10.1212/WNL.0b013e3181ae7cbc.
Schara U, Barisic N, Deschauer M, Lindberg C, Straub V, Strigl-Pill N, et al. Ephedrine therapy in eight patients with congenital myasthenic syndrome due to DOK7 mutations. Neuromuscul Disord. 2009;19(12):828–32. https://doi.org/10.1016/j.nmd.2009.09.008.
Fukudome T, Ohno K, Brengman JM, Engel AG. Quinidine normalizes the open duration of slow-channel mutants of the acetylcholine receptor. Neuroreport. 1998;9(8):1907–11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Constantine Farmakidis has received consultancy fees from Momenta and is an advisory board member of Terumo BCT and Alnylam.
Mamatha Pasnoor is a consultant for Momenta and has served on advisory boards for Terumo BCT and Alexion.
Richard J. Barohn has received speaker fees from NuFactor, Plan 365 Inc., and Option Care, is on the advisory board of Novaritis Pharmaceuticals, and has received grants from Eli Lilly and Company, PTC Therapeutics, Cytokinetics, Inc., Neuraltus Pharmaceuticals, Inc., Alexion Pharmaceuticals, Inc., ALSA, MDA-Myotonic Dystrophy Foundation, The Marigold Foundation, Sarepta Therapeutics, IONIS Pharmaceuticals, TEVA Pharmaceuticals, Biomarin, Sanofi/Genzyme, and the NIH.
Mazen M. Dimachkie is a consultant or on the speaker’s bureau for Alnylam, Audentes, Biomarin, Catalyst, CSL-Behring, Genzyme, Mallinckrodt, Momenta, Novartis, NuFactor, Octapharma, Sanofi, Shire and Terumo. Dr. Dimachkie received grants from Alexion, Alnylam, Amicus, Biomarin, Bristol-Myers Squibb, Catalyst, CSL-Behring, FDA/OPD, GlaxoSmithKline, Genentech, Grifols, MDA, NIH, Novartis, Genzyme, Octapharma, UCB Biopharma, Viromed and TMA.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Neuromuscular Disorders
Rights and permissions
About this article
Cite this article
Farmakidis, C., Pasnoor, M., Barohn, R.J. et al. Congenital Myasthenic Syndromes: a Clinical and Treatment Approach. Curr Treat Options Neurol 20, 36 (2018). https://doi.org/10.1007/s11940-018-0520-7
Published:
DOI: https://doi.org/10.1007/s11940-018-0520-7