Opinion Statement
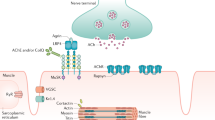
Approximately 5–8 % of myasthenia gravis (MG) patients test positive for antibodies against muscle- specific tyrosine kinase (MuSK) receptors. Except in extremely rare reports, all are acetylcholine receptor (AChR) antibody-negative. While MuSK myasthenia gravis (MMG) patients have distinct clinical phenotypes and may differ from AChR-positive patients in diagnostic testing and response to treatment, goals for the treatment of MMG are similar to those in non-MMG. Priority of treatment should be directed toward reducing weakness as much and as quickly as possible. This is particularly true in patients with bulbar or respiratory weakness in order to avoid progression to respiratory failure. After this initial phase, medications should be slowly tapered to the minimum effective dose. Considering the natural history of MMG, a small proportion of patients can be completely taken off treatment at some point, but the vast majority will require treatment for life. Response to acetylcholinesterase inhibitors (ACEi) is usually poor, and the likelihood of side effects is relatively high. However, considering the benign nature of this line of treatment and the potential for rapid response, an initial trial of ACEi is reasonable. Unless clearly contraindicated by other medical conditions, we recommend initiating corticosteroid treatment for all MMG patients, starting at a dose of 1.5–2 mg /kg/ day of prednisone, followed by gradual and slow taper to the minimum effective dose. A steroid-sparing agent such as azathioprine – and, less often, mycophenolate mofetil or cyclosporine – may be added. When prednisone is used in combination with another immunosuppressive agent, reducing and then tapering off prednisone may be tried after maximum improvement is achieved. It should be emphasized that response to immunosuppressive medications can be delayed for months, although most patients eventually show marked and sustained response. Cyclophosphamide may be used sparingly in select patients who do not respond to the above medications. Rituximab has shown promising results in MMG, and should be considered in severe and refractory cases or in situations where other options are contraindicated or not tolerated by patients. Acute exacerbations may be treated by plasma exchange, which most reports indicate is superior to IVIg, although IVIg may still be used. To date, there is no convincing evidence for the role of thymectomy in MMG.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lindstrom JM. Acetylcholine receptors and myasthenia gravis. Muscle Nerve. 2000;23:453–77.
Lindstrom JM, Seybold ME, Lennon VA, et al. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976;26:1054–9.
Pasnoor M, Wolfe G, Nations S, et al. Clinical findings in MuSK-antibody positive myasthenia gravis: a U.S. experience. Muscle Nerve. 2010;41:370–4.
Guptill JF, Sanders DB, Evoli A. Anti-MuSK antibody myasthenia gravis: clinical finding and response to therapy in two large cohorts. Muscle Nerve. 2011;44:36–40. A retrospective review of 110 MMG patients in two centers in the USA and Italy confirming female predominance and frequent oculobulbar weakness and crises. The response to treatment of various immunomodulatory/immunosuppressive treatments in MMG was evaluated confirming good response to TPE. This study included one of the largest numbers of MMG patients evaluated.
Wolfe GI, Oh SJ. Clinical phenotype of muscle-specific tyrosine kinase antibody positive myasthenia gravis. Ann N Y Acad Sci. 2008;1132:71–5.
Vincent A, Lang B. The prevalence of MuSK antibody positive myasthenia gravis worldwide. J Neuroimmunol. 2006;178:233.
Vincent A, Leite MI, Farrugia ME, et al. Myasthenia gravis seronegative for acetylcholine receptor antibody. Ann N Y Acad Sci. 2008;1132:84–92.
Sanders DB, El-Salem K, Massey JM, et al. Clinical aspects of MuSK antibody positive seronegative MG. Neurology. 2003;60:1978–80.
Hoch W, McConville J, Helms S, et al. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. 2001;7:365–8.
Zhou L, McConville J, Chaudhry V, et al. Clinical comparison of muscle-specific tyrosine kinase (MuSK) antibody-positive and –negative myasthenic patients. Muscle Nerve. 2004;30:55–60.
Deymeer F, Gungor-Tuncer O, et al. Clinical comparison of anti-MuSK- vs antiAChR-positive and seronegative myasthenia gravis. Neurology. 2007;68:609–11.
Bartoccioni E, Scuderi F, Minicuci GM, et al. Anti-MuSK antibodies: correlation with myasthenia gravis severity. Neurology. 2006;67:505–7.
Guptill JT, Sanders DB. Update on muscle-specific tyrosine kinase antibody positive myasthenia gravis. Curr Opin Neurol. 2010;23:530–5.
Evoli A, Padua L. Diagnosis and therapy of myasthenia gravis with antibodies to muscle specific kinase. Autoimmun Rev. 2013;12:913–35. An excellent review of the clinical phenotypes, diagnosis and treatment of MMG by experts in the field.
Evoli A, Alboini PE, Bisonni A, et al. Management challenges in muscle specific tyrosine kinase myasthenia gravis. Ann N Y Acad Sci. 2012;1274:86–91. An excellent review of the current clinical, diagnostic and treatment challenges of MMG patients.
Sanders DB, Juel VC. MuSK-antibody positive myasthenia gravis. Questions from the clinic. J Neuroimmunol. 2008;201–202:85–9.
Evoli A, Bianchi MR, Riso R, et al. Response to therapy in myasthenia gravis with anti-MuSK antibodies. Ann N Y Acad Sci. 2008;1132:76–83.
Lavrnic D, Losen M, Vujic A, et al. The features of myasthenia gravis with autoantibodies to MuSK. J Neurol Neurosurg Psychiatry. 2005;76:1099–102.
Evoli A, Tonali PA, Padua L, et al. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain. 2003;126:2304–11.
Stickler DE, Massey JM, Sanders DB. MuSK-antibody positive myasthenia gravis: clinical and electrodiagnostic patterns. Clin Neurophysiol. 2005;116:2065–8.
Hatanaka Y, Hemmi S, Morgan MB, et al. Nonresponsiveness to anticholinesterase agents in patients with anti-MuSK-antibody-positive MG. Neurology. 2005;65:1508–9.
Oh SJ. Muscle specific receptor tyrosine kinase antibody positive myasthenia gravis current status. J Clin Neurol. 2009;5:53–64.
Kuwabara S, Nemoto Y, Misawa S, et al. Anti-MuSK-positive myasthenia gravis: neuromuscular transmission failure in facial and limb muscles. Acta Neurol Scand. 2007;115:126–8.
Farrugia ME, Kennett RP, Newsom-Davis J, et al. Single-fiber electromyography in limb and facial muscles in muscle-specific kinase antibody and acetylcholine receptor antibody myasthenia gravis. Muscle Nerve. 2006;33:568–70.
Rostedt PA, Ahlqvist K, Bartoccioni E, et al. Neurophysiological and mitochondrial abnormalities in MuSK antibody seropositive myasthenia gravis compared to other immunological subtypes. Clin Neurophysiol. 2006;117:1434–43.
Lauriola L, Ranelletti F, Maggiano N, et al. Thymus changes in anti-MuSK-positive and -negative myasthenia gravis. Neurology. 2005;64:536–8.
Leite MI, Ströbel P, Jones M, et al. Fewer thymic changes in MuSK antibody-positive than in MuSK antibody-negative MG. Ann Neurol. 2005;57:444–8
Saka E, Topcuoglu MA, Akkaya B, et al. Thymus changes in anti-MuSK-positive and -negative myasthenia gravis. Neurology. 2005;65:782–3.
Juel VC, Massey JM. Autoimmune myasthenia gravis: recommendations for treatment and immunologic modulation. Curr Treat Options Neurol. 2005;7:3–14.
Punga AR, Stålberg EV. Acetylcholinesterase inhibitors in MG: to be or not to be? Muscle Nerve. 2009;39:724.
Ionita CM, Acsadi G. Management of juvenile myasthenia gravis. Pediatr Neurol. 2013;48:95.
Chiang LM, Darras BT, Kang PB. Juvenile myasthenia gravis. Muscle Nerve. 2009;39:423.
Punga AR, Flink R, Askmark H, Stålberg EV. Cholinergic neuromuscular hyperactivity in patients with myasthenia gravis seropositive for MuSK antibody. Muscle Nerve. 2006;34:111–5.
Herold MJ, McPherson KG, Reichardt HM. Glucocorticoids in T cell apoptosis and function. Cell Mol Life Sci. 2006;63:60–72.
Rogatsky I, Ivashkiv LB. Glucocorticoid modulation of cytokine signaling. Tissue Antigens. 2006;68:1–12.
Sanders DB, Evoli A. Immunosuppressive treatment of myasthenia gravis. Autoimmunity. 2010;43:428–35.
Schneider-Gold C, Gajdos P, Toyka KV, Hohlfeld RR. Corticosteroids for myasthenia gravis. Cochrane Database Syst Rev. 2005; 18(2) (Apr).
Richman DP, Agius MA. Treatment of autoimmune myasthenia gravis. Neurology. 2003;61:1652–61.
Pascuzzi RM, Coslett HB, Johns TR. Long-term corticosteroid treatment of myasthenia gravis: report of 116 patients. Ann Neurol. 1984;15:291–8.
Miller RG, Milner-Brown HS, Mirka A. Prednisone induced worsening of neuromuscular function in myasthenia gravis. Neurology. 1986;36:729–32.
Mantegazza R, Antozzi C, Peluchetti D, et al. Azathioprine as a single drug or in combination with steroids in the treatment of myasthenia gravis. J Neurol. 1988;235:449–53.
Sathasivam S. Steroids and immunosuppressant drugs in myasthenia gravis. Nat Clin Pract Neurol. 2008;4:317–27.
Palace J, Newsom-Davis J, Lecky B. A randomized double-blind trial of prednisolone alone or with azathioprine in myasthenia gravis. Myasthenia Gravis Study Group. Neurology. 1998;50:1778–83.
Skeie GO, Apostolski S, Evoli A, et al. Guidelines for treatment of autoimmune neuromuscular transmission disorders. Eur J Neurol. 2010;17:893–902.
Silvestri NJ, Wolfe GI. Myasthenia gravis. Semin Neurol. 2012;32:215–26.
Lennard L. Implementation of TPMT testing. Br J Clin Pharmacol. 2013, Aug 21. doi:10.1111/bcp.12226.
Kissel JT, Levy RJ, Mendell JR, Griggs RC. Azathioprine toxicity in neuromuscular disease. Neurology. 1986;36:35–9.
Herrlinger U, Weller M, Dichgans J, Melms A. Association of primary central nervous system lymphoma with long-term azathioprine therapy for myasthenia gravis. Ann Neurol. 2000;47:682–3.
Chaudhry V, Cornblath DR, Griffin JW, et al. Mycophenolate mofetil: a safe and promising immunosuppressant in neuromuscular diseases. Neurology. 2001;56:94–6.
Ciafaloni E, Massey JM, Tucker-Lipscomb B, Sanders DB. Mycophenolate mofetil for myasthenia gravis: an open-label pilot study. Neurology. 2001;56:97–9.
Meriggioli MN, Ciafaloni E, Al-Hayk KA, et al. Mycophenolate mofetil in the treatment of myasthenia gravis: an analysis of efficacy, safety and tolerability. Neurology. 2003;61:1438–40.
Hehir MK, Burns TM, Alpers JP, et al. Mycophenolate mofetil in AChR-antibody positive myasthenia gravis: outcomes in 102 patients. Muscle Nerve. 2010;41:593–8.
The Muscle Study Group. A trial of mycophenolate mofetil with prednisone as initial immunotherapy in myasthenia gravis. Neurology. 2008;71:394–9.
Sanders DB, McDermott M, Thornton C, et al. A trial of mycophenolate mofetil with prednisone as initial immunotherapy in myasthenia gravis. Neurology. 2008;71:394–9.
Sanders DB, Siddiqi ZA. Lessons from two trials of mycophenolate mofetil in myasthenia gravis. Ann N Y Acad Sci. 2008;1132:249–53.
Vernino S, Salomao DR, Habermann TM, O'Neill BP. Primary CNS lymphoma complicating treatment of myasthenia gravis with mycophenolate mofetil. Neurology. 2005;65:639–41.
Neff RT, Hurst FP, Falta EM, et al. Progressive multifocal leukoencephalopathy and use of mycophenolate mofetil after kidney transplantation. Transplantation. 2008;86:1474–8.
Tindall RSA, Rollins JA, Phillips JT, et al. Preliminary results of a double-blind, randomized, placebo-controlled trial of cyclosporine in myasthenia gravis. N Engl J Med. 1987;316:719–24.
Tindall RSA, Phillips JT, Rollins JA, et al. A clinical therapeutic trial of cyclosporine in myasthenia gravis. Ann N Y Acad Sci. 1993;681:539–51.
Ciafaloni E, Nikhar NK, Massey JM, Sanders DB. Retrospective analysis of the use of cyclosporine in myasthenia gravis. Neurology. 2000;55:448–50.
Rappaport DC, Weisbrod GL, Herman SJ. Cyclosporine-induced lymphoma following a unilateral lung transplant. The Toronto Lung Transplant Group. Can Assoc Radiol J. 1989;40:110–1.
Perez MC, Buot WL, Mercado-Danguilan C, et al. Stable remissions in myasthenia gravis. Neurology. 1981;31:32–7.
De Feo LG, Schottlender J, Martelli NA, Molfino NA. Use of intravenous pulsed cyclophosphamide in severe, generalized myasthenia gravis. Muscle Nerve. 2002;26:31–6.
Drachman DB, Jones RJ, Brodsky RA. Treatment of refractory myasthenia: “rebooting” with high-dose cyclophosphamide. Ann Neurol. 2003;53:29–34.
Drachman DB, Adams RN, Hu R, et al. Rebooting the immune system with high-dose cyclophosphamide for treatment of refractory myasthenia gravis. Ann N Y Acad Sci. 2008;1132:305–14.
Lin PT, Martin BA, Weinacker AB, et al. High-dose cyclophosphamide in refractory myasthenia gravis with MuSK antibodies. Muscle Nerve. 2006;33:433–5.
Talar-Williams C, Hijazi YM, Walther MM, et al. Cyclophosphamide-induced cystitis and bladder cancer in patients with Wegener Granulomatosis. Ann Intern Med. 1996;124:477–84.
Hain B, Jordan K, Deschauer M, Zierz S. Successful treatment of MuSK antibody-positive myasthenia gravis with rituximab. Muscle Nerve. 2006;33:575–80.
Blum S, Gillis D, Brown H, et al. Use and monitoring of low dose rituximab in myasthenia gravis. J Neurol Neurosurg Psychiatry. 2011;82:659–63.
Díaz-Manera J, Martínez-Hernández E, Querol L, et al. Long-lasting treatment effect of rituximab in MuSK myasthenia. Neurology. 2012;78:189–93. A clinical study on 17 MG patients, 6 of whom are MMG, demonstrating long-lasting response to treatment with Rituximab, and recommending this medication as an early option for those not responding to corticosteroids.
Silvestri NJ, Wolfe GI. Myasthenia gravis. Semin Neurol. 2012;32:215–26.
Yi JS, Decroos EC, Sanders DB, et al. Prolonged B-cell depletion in MuSK myasthenia gravis following rituximab treatment. Muscle Nerve. 2013;48:992–3.
Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9:425–37.
Wolfe GI, Trivedi JR, Oh SJ. Clinical review of muscle-specific tyrosine kinase-antibody positive myasthenia gravis. J Clin Neuromuscul Disord. 2007;8:217–24.
Kaplan AA. Therapeutic plasma exchange: a technical and operational review. J Clin Apher. 2013;28:3–10.
Kalantari K. The choice of vascular access for therapeutic apheresis. J Clin Apher. 2012;27:153–9.
Gurland HJ, Lysaght MJ, Samtleben W, Schmidt B. Comparative evaluation of filters used in membrane plasmapheresis. Nephron. 1984;36:173–82.
Gajdos P, Chevret S, Toyka K. Plasma exchange for generalized myasthenia gravis. Cochrane Database Syst Rev. 2002; Oct 21.
Juel VC. Myasthenia gravis: management of myasthenic crisis and perioperative care. Semin Neurol. 2004;24:75–81.
Ebadi H, Barth D, Bril V. Safety of plasma exchange therapy in patients with myasthenia gravis. Muscle Nerve. 2013;47:510–4.
Sutton DMC, Nair RC, Rock NG, Canadian Apheresis Study Group. Complications of plasma exchange. Transfusion. 1989;29:124.
Basic-Jukic N, Kes P, Glavas-Boras S, et al. Complications of therapeutic plasma exchange: experience with 4857 treatments. Ther Apher Dial. 2005;9:391–5.
Guptill JT, Oakley D, Kuchibhatla M, et al. Retrospective study of complications of therapeutic plasma exchange in myasthenia. Muscle Nerve. 2013;47:170–6.
Mandawat A, Kaminski HJ, Cutter G, et al. Comparative analysis of therapeutic options used for myasthenia gravis. Ann Neurol. 2010;68:797–805.
Guptill JT, Sharma BK, Marano A, et al. Estimated cost of treating myasthenia gravis in an insured U.S. population. Muscle Nerve. 2012;45:363–6.
Winters JL, Brown D, Hazard E, et al. Cost-minimization analysis of the direct costs of TPE and IVIg in the treatment of Guillain-Barré syndrome. BMC Health Serv Res. 2011;11:101.
Heatwole C, Johnson N, Holloway R, Noyes K. Plasma exchange versus intravenous immunoglobulin for myasthenia gravis crisis: an acute hospital cost comparison study. J Clin Neuromuscul Dis. 2011;13:85–94.
Elovaara I, Apostolski S, van Doorn P, et al. EFNS guidelines for the use of intravenous immunoglobulin in treatment of neurological diseases: EFNS task force on the use of intravenous immunoglobulin in treatment of neurological diseases. Eur J Neurol. 2008;15:893–908.
Burks AW, Sampson HA, Buckley RH. Anaphylactic reactions after gamma globulin administration in patients with hypogammaglobulinemia. N Engl J Med. 1986;314:560–4.
Rachid R, Bonilla FA. The role of anti-IgA antibodies in causing adverse reactions to gamma globulin infusion in immunodeficient patients: a comprehensive review of the literature. J Allergy Clin Immunol. 2012;129:628–34.
Nadeau JA, Bhibhatbhan A, McDougall D, et al. Identification and comparison of adverse events for preparations of IVIG in patients with neuromuscular disorders. Clin Neurol Neurosurg. 2010;112:467–9.
Dantal J. Intravenous immunoglobulins: in-depth review of excipients and acute kidney injury risk. Am J Nephrol. 2013;38:275–84.
Katz U, Achiron A, Sherer Y, Shoenfeld Y. Safety of intravenous immunoglobulin (IVIG) therapy. Autoimmun Rev. 2007;6:257–9.
Jaretzki III A, Steinglass KM, Sonett JR. Thymectomy in the management of myasthenia gravis. Semin Neurol. 2004;24:49–62.
Bulkley GB, Bass KN, Stephenson GR, et al. Extended cervicomediastinal thymectomy in the integrated management of myasthenia gravis. Ann Surg. 1997;226:324–34.
Mori S, Kishi M, Kubo S, et al. 3,4-Diaminopyridine improves neuromuscular transmission in a MuSK antibody-induced mouse model of myasthenia gravis. J Neuroimmunol. 2012;245:75–8.
Ponseti JM, Gamez J, Azem J, et al. Tacrolimus for myasthenia gravis a clinical study of 212 patients. Ann N Y Acad Sci. 2008;1132:254–63.
Ponseti JM, Azem J, Fort JM, et al. Benefits of FK506 (tacrolimus) for residual, cyclosporine- and prednisone-resistant myasthenia gravis: one-year follow up of an open-label study. Clin Neurol Neurosurg. 2005;107:187–90.
Ponseti JM, Azem J, Fort JM, et al. Long-term results of tacrolimus in cyclosporine- and prednisone dependent myasthenia gravis. Neurology. 2005;64:1641–3.
Ponseti JM, Gamez J, Azem J, et al. Post-thymectomy combined treatment of prednisone and tacrolimus versus prednisone alone for consolidation of complete stable remission in patients with myasthenia gravis: a non-randomized, non-controlled study. Curr Med Res Opin. 2007;23:1269–78.
Compliance with Ethics Guidelines
Conflict of Interest
Khalid El-Salem, Ahmed Yassin, Kefah Al-Hayk, Salma Yahya, Duha Al-Shorafat, and Said S. Dahbour declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Neuromuscular Disorders
Rights and permissions
About this article
Cite this article
El-Salem, K., Yassin, A., Al-Hayk, K. et al. Treatment of MuSK-Associated Myasthenia Gravis. Curr Treat Options Neurol 16, 283 (2014). https://doi.org/10.1007/s11940-014-0283-8
Published:
DOI: https://doi.org/10.1007/s11940-014-0283-8