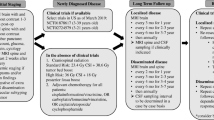
Opinion statement
In the past three decades, the survival for patients with medulloblastoma has improved remarkably. Contemporary “standard” therapy for children with medulloblastoma consists of maximal surgical resection followed by craniospinal irradiation with a boost to the posterior fossa, combined with adjuvant chemotherapy. The use of such multimodal therapeutic approaches results in progression-free survival (PFS) rates of 75% to 80% for patients with average-risk disease and approximately 60% for highrisk patients. However, despite the marked improvements in survival, many therapeutic challenges remain. Children with macroscopic metastatic disease (M2/M3) at presentation continue to fare poorly, with the best reports only attaining PFS rates up to 40%. Furthermore, despite intensive multimodal therapy, some patients have disease progression or recurrence, which for most remains incurable. The early recognition of these patients is imperative in order to institute treatment modifications, such as intensification and/or the use of novel experimental therapies. Additionally, the price for cure is clearly evident in survivors, who suffer from significant, often debilitating long-term neurocognitive and neuroendocrine sequela. Using the current clinical stratification system, a significant number of patients are overtreated and unnecessarily subjected to these long-term toxicities. This group of patients would benefit from reductions in therapy. Refinements in patient stratification and further improvement in outcome are unlikely to be achieved without improved knowledge of tumor biology. Several molecular alterations have already been identified, many of which appear to have prognostic significance. Furthermore, the disruption of molecular alterations in signaling pathways involved in the development and maintenance of medulloblastoma using novel molecularly targeted therapies promises to improve outcomes and reduce toxicity for patients with medulloblastoma. It is envisaged that in the near future children diagnosed with medulloblastoma will be more accurately stratified based on a combination of clinical variables and molecular profiles. Improved risk stratification will permit delivery of individualized therapy using conventional treatment modalities in conjunction with novel targeted therapeutic approaches.
Similar content being viewed by others
References and Recommended Reading
Thomas PR, Deutsch M, Kepner JL, et al.: Low-stage medulloblastoma: final analysis of trial comparing standard-dose with reduced-dose neuraxis irradiation. J Clin Oncol 2000, 18:3004–3011.
Packer RJ, Goldwein J, Nicholson HS, et al.: Treatment of children with medulloblastoma with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: a Children’s Cancer Group study. J Clin Oncol 1999, 17:2127–2136. Seminal study that demonstrated the efficacy of delivering decreased-dose CSI (23.4 Gy) for average-risk patients, when combined with adjuvant chemotherapy given during and after radiotherapy.
Strother D, Ashley D, Kellie SJ, et al.: Feasibility of four consecutive high-dose chemotherapy cycles with stem-cell rescue for patients with newly diagnosed medulloblastoma or supratentorial PNET after craniospinal radiotherapy: results of a collaborative study. J Clin Oncol 2001, 19:2696–2704.
Kortmann RD, Kuhl J, Timmermann B, et al.: Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood, results of the German prospective randomized trial HIT ’91. Int J Radiat Oncol Biol Phys 2000, 46:269–279.
Taylor RE, Bailey CC, Robinson K, et al.: Results of a randomized study of pre-radiation chemotherapy vs radiotherapy alone for non-metastatic (M0-M1) medulloblastoma. The SIOP/UKCCSG PNET-3 study. J Clin Oncol 2003, 21:1581–1591.
Carrie C, Hoffstetter S, Gomez F, et al.: Impact of targeting deviations on outcome in medulloblastoma: study of the French Society of Pediatric Oncology (SFOP). Int J Radiat Oncol Biol Phys 1999, 45:435–439.
Zeltzer PM, Boyett JM, Finlay JL, et al.: Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. J Clin Oncol 1999, 17:832–845.
Taylor RE, Bailey CC, Robinson K, et al.: Outcome for patients with metastatic (M2-3) medulloblastoma treated with SIOP/UKCCSG PNET-3 chemotherapy. Eur J Cancer 2005, 41:727–734.
Gajjar A, Chintagumpala M, Kellie S, et al.: Excellent event free survival in newly diagnosed high risk medulloblastoma treated with craniospinal radiation therapy followed by 4 cycles of high-dose chemotherapy and stem cell rescue: results of a prospective multicenter trial (SJMB 96). Paper presented at the 11th International Symposium on Pediatric Neuro-oncology. Boston, MA, June 13–16, 2004
Pomeroy SL, Tamayo P, Gaasenbeek M, et al.: Prediction of central nervous system embryonal tumour outcome based on gene expression. Nature 2002, 415:436–442. Gene expression profiles obtained using oligonucleotide microarrays revealed that medulloblastomas are molecularly distinct from other embryonal tumors (supratentorial primitive neuroectodermal tumors and atypical teratoid rhabdomyosarcoma). Furthermore, outcome prediction based on a small panel of genes more accurately stratified patients according to outcome than did stratification based on clinical variables.
Mulhern RK, Palmer SL, Merchant TE, et al.: Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol 2005, 23:5511–5519. Prospective longitudinal study that compared the effects of risk-adapted CSI dose (23.4 vs 36 Gy) on IQ and academic achievement. All patients were treated with the same adjuvant chemotherapy. Average-risk and high-risk patients revealed a decrease in mean IQ (0.99 vs 3 IQ points/year), with the greatest decreases seen in younger (< 7 years) patients (2.41 and 3.71 IQ points/year for average-risk and high-risk patients, respectively). These findings support the notion that CSI dose reductions for patients with average-risk disease result in increased preservation of neurocognitive function.
Pollack IF, Polinko P, Albright AL, et al.: Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. Neurosurgery 1995, 37:885–893.
Huber JF, Bradley K, Spiegler BJ, Dennis M: Long-term effects of transient cerebellar mutism after cerebellar astrocytoma or medulloblastoma tumor resection in childhood. Childs Nerv Syst 2006, 22:132–138.
Mulhern RK, Kepner JL, Thomas PR, et al.: Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduceddose craniospinal irradiation: a Pediatric Oncology Group Study. J Clin Oncol 1998, 16:1723–1728.
Spoudeas HA: Growth and endocrine function after chemotherapy and radiotherapy in childhood. Eur J Cancer 2002, 38:1748–1759.
Ris MD, Packer RJ, Goldwein J, et al.: Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children’s Cancer Group study. J Clin Oncol 2001, 19:3470–3476.
Goldwein JW, Radcliffe J, Johnson J, et al.: Updated results of a pilot study of low dose craniospinal irradiation plus chemotherapy for children under five with cerebellar primitive neuroectodermal tumors (medulloblastoma). Int J Radiat Oncol Biol Phys 1996, 34:899–904.
Jakacki RI, Feldman H, Jamison C, et al.: A pilot study of preirradiation chemotherapy and 1800 cGy craniospinal irradiation in young children with medulloblastoma. Int J Radiat Oncol Biol Phys 2004, 60:531–636.
Bouffet E, Gentet JC, Doz F, et al.: Metastatic medulloblastoma: the experience of the French Cooperative M7 Group. Eur J Cancer 1994, 30A:1478–1483.
Prados MD, Wara W, Edwards MS, et al.: Treatment of high-risk medulloblastoma and other primitive neuroectodermal tumors with reduced dose craniospinal radiation therapy and multi-agent nitrosourea-based chemotherapy. Pediatr Neurosurg 1996, 25:174–181.
Wolden SL, Dunkel IJ, Souweidane MM, et al.: Patterns of failure using a conformal radiation therapy tumor bed boost for medulloblastoma. J Clin Oncol 2003, 21:3079–3083.
Merchant TE, Kun LE, Krasin MJ, et al.: A multi-institutional prospective trial of reduced-dose craniospinal irradiation (23.4 Gy) followed by conformal posterior fossa (36 Gy) and primary site irradiation (55.8 Gy) and dose intensive chemotherapy for average-risk medulloblastoma. Int J Radiat Oncol Biol Phys 2003, 57(Suppl 1):S194-S195.
Prados MD, Edwards MS, Chang SM, et al.: Hyperfractionated craniospinal radiation therapy for primitive neuroectodermal tumors: results of a phase II study. Int J Radiat Oncol Biol Phys 1999, 43:279–285.
Carrie C, Muracciole X, Gomez F, et al.: French Society of Pediatric Oncology. Conformal radiotherapy, reduced boost volume, hyperfractionated radiotherapy, and online quality control in standard-risk medulloblastoma without chemotherapy: results of the French M-SFOP 98 protocol. Int J Radiat Oncol Biol Phys 2005, 63:711–716.
Geyer JR, Sposto R, Jennings M, et al.: Children’s Cancer Group. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children’s Cancer Group. J Clin Oncol 2005, 23:7621–7631.
Grill J, Sainte-Rose C, Jouvet A, et al.: French Society of Paediatric Oncology. Treatment of medulloblastoma with postoperative chemotherapy alone: an SFOP prospective trial in young children. Lancet Oncol 2005, 6:573–580.
Rutkowski S, Bode U, Deinlein F, et al.: Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med 2005, 352:978–986. This study demonstrated that chemotherapy intensified with intravenous and intrathecal methotrexate without radiotherapy prevents disease progression in 82% of infants with average-risk features. However, 83% (19/23) of evaluable patients had evidence of leukoencephalopathy, and the mean IQ of survivors was significantly lower than that of age-matched controls.
Fouladi M, Gajjar A, Boyett JM, et al.: Comparison of CSF cytology and spinal magnetic resonance imaging in the detection of leptomeningeal disease in pediatric medulloblastoma or primitive neuroectodermal tumor. J Clin Oncol 1999, 17:3234–3237.
Blaney SM, Boyett J, Friedman H, et al.: Phase I clinical trial of mafosfamide in infants and children aged 3 years or younger with newly diagnosed embryonal tumors: a pediatric brain tumor consortium study (PBTC-001). J Clin Oncol 2005, 23:525–531.
Hallahan AR, Pritchard JI, Chandraratna RA, et al.: BMP-2 mediates retinoid-induced apoptosis in medulloblastoma cells through a paracrine effect. Nat Med 2003, 9:1033–1038. Retinoids induced extensive apoptosis of medulloblastoma cells and caused tumor regression in xenograft models. The gene BMP-2 was discovered to be the principal mediator of these effects.
Thompson MC, Fuller C, Hogg TL, et al.: Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol 2006, In press. The authors used oligonucleotide microarrays to generate gene-expression profiles from a panel of 46 medulloblastomas. They identified gene expression signatures that distinguished patients according to specific subgroups governed by the presence of mutations in certain cell signaling pathways (eg, the WNT and SHH pathways). Mutation-specific gene expression signatures have important clinical implications for the future design of clinical trials using molecular-targeted therapies, by assisting in the identification of patients more likely to respond to inhibitors of pathways deregulated in their tumors. Another finding was the strong association between the loss of chromosome 6 (monosomy 6) and the presence of activating mutations in the gene CTNNB (which encodes for the protein β-catenin). This discovery provides important insights into potential cooperating mutations that may lead to the development of medulloblastoma.
Gilbertson RJ: ERBB2 in pediatric cancer: innocent until proven guilty. Oncologist 2005, 10:508–517.
Gajjar A, Hernan R, Kocak M, et al.: Clinical, histopathologic, and molecular markers of prognosis: toward a new disease risk stratification system for medulloblastoma. J Clin Oncol 2004, 22:984–993. The authors show that only one patient with average-risk medulloblastoma and ERBB2 negativity (n = 26) experienced disease progression. In contrast, disease progression was observed in 46% of average-risk patients who were ERBB2 positive (n = 13). This study provides strong evidence for including molecular markers in the risk stratification of patients with medulloblastoma and also demonstrates the feasibility of rapid molecular analysis of specimens from multiple institutions in a central reference laboratory.
Warren KE: Molecularly targeted therapy for pediatric brain tumors. J Neurooncol 2005, 75:335–343.
Ellison DW, Onilude OE, Lindsey JC, et al.: United Kingdom Children’s Cancer Study Group Brain Tumour Committee. beta-Catenin status predicts a favorable outcome in childhood medulloblastoma. J Clin Oncol 2005, 23:7951–7957. This retrospective analysis of 109 patients with medulloblastoma revealed a significantly increased survival (P = 0.0015) associated with high nuclear β-catenin immunoreactivity. An interesting observation was that all patients with high nuclear β-catenin and adverse prognostic features, including metastasis (n = 3) and the large cell/anaplastic histopathological variants (n = 4), are longterm survivors. Hence, β-catenin appears to denote a subgroup of patients particularly responsive to treatment, even among patients with unfavorable prognostic features.
Moon RT, Kohn AD, De Ferrari GV, Kaykas A: WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet 2004, 5:691–701.
Gilbertson RJ: Medulloblastoma: signaling a change in treatment. Lancet Oncol 2004, 5:209–218.
Romer JT, Kimura H, Magdaleno S, et al.: Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/-)p53(-/-) mice. Cancer Cell 2004, 6:229–240. In a medulloblastoma mouse model (Ptc1 +/-) in which the SHH pathway was deregulated, a small-molecule inhibitor of SHH signaling (specifically targeting and binding to smoothened) efficiently suppressed tumor cell proliferation and caused complete tumor regression, resulting in prolonged survival in mice. These results provide a strong rationale for the investigation of this inhibitor in clinical trials.
MacDonald TJ, Brown KM, LaFleur B, et al.: Expression profiling of medulloblastoma: PDGFRA and the RAS/ MAPK pathway as therapeutic targets for metastatic disease. Nat Genet 2001, 29:143–152.
Gilbertson RJ, Clifford SC: PDGFRB is overexpressed in metastatic medulloblastoma. Nat Genet 2003, 35:197–198.
Hallahan AR, Pritchard JI, Hansen S, et al.: The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehoginduced medulloblastomas. Cancer Res 2004, 64:7794–7800.
Fan X, Mikolaenko I, Elhassan I, et al.: Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res 2004, 64:7787–7793.
Nickoloff BJ, Osborne BA, Miele L: Notch signaling as a therapeutic target in cancer: a new approach to the development of cell fate modifying agents. Oncogene 2003, 22:6598–6608.
Aldosari N, Bigner SH, Burger PC, et al.: MYCC and MYCN oncogene amplification in medulloblastoma. A fluorescence in situ hybridization study on paraffin sections from the Children’s Oncology Group. Arch Pathol Lab Med 2002, 126:540–544.
Eberhart CG, Kratz J, Wang Y, et al.: Histopathological and molecular prognostic markers in medulloblastoma: c-myc, N-myc, TrkC, and anaplasia. J Neuropathol Exp Neurol 2004, 63:441–449.
Herms J, Neidt I, Luscher B, et al.: C-MYC expression in medulloblastoma and its prognostic value. Int J Cancer 2000, 89:395–402.
Stearns D, Chaudhry A, Abel TW, et al.: c-myc overexpression causes anaplasia in medulloblastoma. Cancer Res 2006, 66:673–681.
Lu X, Pearson A, Lunec J: The MYCN oncoprotein as a drug development target. Cancer Lett 2003, 197:125–130.
Grotzer MA, Janss AJ, Fung K, et al.: TrkC expression predicts good clinical outcome in primitive neuroectodermal brain tumors. J Clin Oncol 2000, 18:1027–1035.
Frank AJ, Hernan R, Hollander A, et al.: Disruption of the TP53 pathway is a frequent event in medulloblastoma and allows ERBB2 overexpression. Brain Res Mol Brain Res 2004, 121:137–140.
Burns AS, Jaros E, Cole M, et al.: The molecular pathology of p53 in primitive neuroectodermal tumours of the central nervous system. Br J Cancer 2002, 86:1117–1123.
Ellison DW, Clifford SC, Gajjar A, Gilbertson RJ: What’s new in neuro-oncology? Recent advances in medulloblastoma. Eur J Pediatr Neurol 2003, 7:53–66.
Gilbertson R: Paediatric embryonic brain tumours: biological and clinical relevance of molecular genetic abnormalities. Eur J Cancer 2002, 38:675–685.
Pan E, Pellarin M, Holmes E, et al.: Isochromosome 17q is a negative prognostic factor in poor-risk childhood medulloblastoma patients. Clin Cancer Res 2005, 11:4733–4740.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gottardo, N.G., Gajjar, A. Current therapy for medulloblastoma. Curr Treat Options Neurol 8, 319–334 (2006). https://doi.org/10.1007/s11940-006-0022-x
Issue Date:
DOI: https://doi.org/10.1007/s11940-006-0022-x