Abstract
Purpose of review
With the advent of biologic therapies for the treatment of inflammatory bowel disease, the roles of thiopurines have continued to evolve. This review will focus on recent advances in pharmacology and the safety and efficacy of thiopurines as maintenance therapies for steroid-induced remissions and post-surgical maintenance of remission and as combination therapies to reduce immunogenicities of biologic agents.
Recent findings
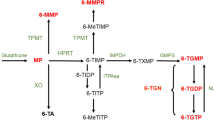
Due to pharmacogenetics of thiopurine S-methyltransferase, thiopurine dosing is more effectively based on monitoring of thiopurine metabolites rather than weight-based dosing. Thiopurines continue to have a role as maintenance therapy after steroid-induced remissions and in combination with biologics to induce and maintain remission. Safety monitoring includes measurements of blood counts, liver chemistries, and dermatologic evaluations and protection from sun exposure.
Summary
Thiopurines appear to be safe during pregnancies and while very uncommon, lymphomas (including hepatosplenic T cell lymphomas) remain a recognized risk, particularly in younger and older males.
Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hindorf U, Lindqvist M, Peterson C, Söderkvist P, Ström M, Hjortswang H, et al. Pharmacogenetics during standardised initiation of thiopurine treatment in inflammatory bowel disease. Gut. 2006;55:1423–31.
Eklund BI, Moberg M, Bergquist J, Mannervik B. Divergent activities of human glutathione transferases in the bioactivation of azathioprine. Mol Pharmacol. 2006;70:747–54.
Amin J, Huang B, Yoon J, Shih DQ. Update 2014: advances to optimize 6-mercaptopurine and azathioprine to reduce toxicity and improve efficacy in the management of IBD. Inflamm Bowel Dis. 2015;21:445–52.
Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980;32:651–62.
Dubinsky MC, Vasiliauskas EA, Singh H, Abreu MT, Papadakis KA, Tran T, et al. 6-Thioguanine can cause serious liver injury in inflammatory bowel disease patients. Gastroenterology. 2003;125:298–303.
Derijks LJ, Gilissen LP, Engels LG, et al. Pharmacokinetics of 6-mercaptopurine in patients with inflammatory bowel disease: implications for therapy. Ther Drug Monit. 2004;26:311–8.
Gardiner SJ, Gearry RB, Begg EJ, Zhang M, Barclay ML. Thiopurine dose in intermediate and normal metabolizers of thiopurine methyltransferase may differ three-fold. Clin Gastroenterol Hepatol. 2008;6:654–60 quiz 604.
•• Walker GJ, Harrison JW, Heap GA, et al. Association of genetic variants in NUDT15 with thiopurine-induced myelosuppression in patients with inflammatory bowel disease. JAMA. 2019;321:773–85 Identification of a new genetic variant in thiopurine metabolism associated with an increased risk of myelosuppression with thiopurine use.
Cuffari C, Dassopoulos T, Turnbough L, Thompson RE, Bayless TM. Thiopurine methyltransferase activity influences clinical response to azathioprine in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2004;2:410–7.
Dassopoulos T, Dubinsky MC, Bentsen JL, Martin CF, Galanko JA, Seidman EG, et al. Randomised clinical trial: individualised vs. weight-based dosing of azathioprine in Crohn’s disease. Aliment Pharmacol Ther. 2014;39:163–75.
•• Yarur AJ, Gondal B, Hirsch A, et al. Higher thioguanine nucleotide metabolite levels are associated with better long-term outcomes in patients with inflammatory bowel diseases. J Clin Gastroenterol. 2018;52:537–44 Updated confirmation of thioguanine metabolite levels as predictor of response than weight-based dosing.
Present DH, Korelitz BI, Wisch N, Glass JL, Sachar DB, Pasternack BS. Treatment of Crohn’s disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med. 1980;302:981–7.
Sandborn WJ, Tremaine WJ, Wolf DC, et al. Lack of effect of intravenous administration on time to respond to azathioprine for steroid-treated Crohn’s disease. North American Azathioprine Study Group. Gastroenterology. 1999;117:527–35.
Panes J, Lopez-Sanroman A, Bermejo F, et al. Early azathioprine therapy is no more effective than placebo for newly diagnosed Crohn’s disease. Gastroenterology. 2013;145:766–74.e1.
Chande N, Townsend CM, Parker CE, et al. Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2016;10:Cd000545.
Jewell DP, Truelove SC. Azathioprine in ulcerative colitis: final report on controlled therapeutic trial. Br Med J. 1974;4:627–30.
Khan KJ, Dubinsky MC, Ford AC, Ullman TA, Talley NJ, Moayyedi P. Efficacy of immunosuppressive therapy for inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011;106:630–42.
Chande N, Tsoulis DJ, MacDonald JK. Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2013;Cd000545.
D'Haens G, Geboes K, Rutgeerts P. Endoscopic and histologic healing of Crohn’s (ileo-) colitis with azathioprine. Gastrointest Endosc. 1999;50:667–71.
Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn’s disease on the need for intestinal surgery. Gut. 2005;54:237–41.
Vernier-Massouille G, Balde M, Salleron J, et al. Natural history of pediatric Crohn’s disease: a population-based cohort study. Gastroenterology. 2008;135:1106–13.
Gisbert JP, Linares PM, McNicholl AG, et al. Meta-analysis: the efficacy of azathioprine and mercaptopurine in ulcerative colitis. Aliment Pharmacol Ther. 2009;30:126–37.
Cohen RD, Stein R, Hanauer SB. Intravenous cyclosporin in ulcerative colitis: a five-year experience. Am J Gastroenterol. 1999;94:1587–92.
Miyake N, Ando T, Ishiguro K, et al. Azathioprine is essential following cyclosporine for patients with steroid-refractory ulcerative colitis. World J Gastroenterol. 2015;21:254–61.
Singh H. Bernstein CN. Clin Gastroenterol Hepatol: Sorting through the risks and benefits of thiopurine therapy for inflammatory bowel diseases; 2019.
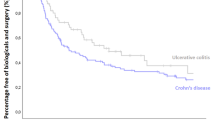
• Eriksson C, Rundquist S, Cao Y, et al. Impact of thiopurines on the natural history and surgical outcome of ulcerative colitis: a cohort study. Gut. 2019;68:623–32 Additional support for efficacy of thiopurines to prevent long-term complications of ulcerative colitis.
Hawthorne AB, Logan RF, Hawkey CJ, Foster PN, Axon AT, Swarbrick ET, et al. Randomised controlled trial of azathioprine withdrawal in ulcerative colitis. Bmj. 1992;305:20–2.
Bouhnik Y, Lemann M, Mary JY, et al. Long-term follow-up of patients with Crohn’s disease treated with azathioprine or 6-mercaptopurine. Lancet. 1996;347:215–9.
Treton X, Bouhnik Y, Mary JY, Colombel JF, Duclos B, Soule JC, et al. Azathioprine withdrawal in patients with Crohn’s disease maintained on prolonged remission: a high risk of relapse. Clin Gastroenterol Hepatol. 2009;7:80–5.
Boyapati RK, Torres J, Palmela C, et al. Withdrawal of immunosuppressant or biologic therapy for patients with quiescent Crohn’s disease. Cochrane Database Syst Rev. 2018;5:Cd012540.
Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh DG, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56:1232–9.
Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–85.
Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–95.
Panaccione R, Ghosh S, Middleton S, Márquez JR, Scott BB, Flint L, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014;146:392–400.e3.
• Colombel JF, Adedokun OJ, Gasink C, et al. Combination therapy with infliximab and azathioprine improves infliximab pharmacokinetic features and efficacy: a post hoc analysis. Clin Gastroenterol Hepatol. 2019;17(8):1525–32 Post hoc analysis of SONIC study suggesting that azathioprine may be efficacious in combination with inflximab by raising blood levels of the biologic in contrast to mechanistic synergy.
Yarur AJ, Kubiliun MJ, Czul F, et al. Concentrations of 6-thioguanine nucleotide correlate with trough levels of infliximab in patients with inflammatory bowel disease on combination therapy. Clin Gastroenterol Hepatol. 2015;13:1118–24.e3.
Matsumoto T, Motoya S, Watanabe K, Hisamatsu T, Nakase H, Yoshimura N, et al. Adalimumab monotherapy and a combination with azathioprine for Crohn’s disease: a prospective, randomized trial. J Crohns Colitis. 2016;10:1259–66.
Kopylov U, Al-Taweel T, Yaghoobi M, et al. Adalimumab monotherapy versus combination therapy with immunomodulators in patients with Crohn’s disease: a systematic review and meta-analysis. J Crohns Colitis. 2014;8:1632–41.
Feagan BG, Chande N, MacDonald JK. Are there any differences in the efficacy and safety of different formulations of Oral 5-ASA used for induction and maintenance of remission in ulcerative colitis? Evidence from Cochrane reviews. Inflamm Bowel Dis. 2013;19:2031–40.
Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–21.
Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–60.
Al-Bawardy B, Ramos GP, Willrich MAV, et al. Vedolizumab drug level correlation with clinical remission, biomarker normalization, and mucosal healing in inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(3):580–6.
Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011;106:685–98.
Baert F, Noman M, Vermeire S, van Assche G, D' Haens G, Carbonez A, et al. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn’s disease. N Engl J Med. 2003;348:601–8.
• Ungar B, Kopylov U, Engel T, et al. Addition of an immunomodulator can reverse antibody formation and loss of response in patients treated with adalimumab. Aliment Pharmacol Ther. 2017;45:276–82 Additional evidence that thiopurines reduce immunogenicity to biologic therapies.
Ben-Horin S, Waterman M, Kopylov U, et al. Addition of an immunomodulator to infliximab therapy eliminates antidrug antibodies in serum and restores clinical response of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:444–7.
van Schaik T, Maljaars JP, Roopram RK, et al. Influence of combination therapy with immune modulators on anti-TNF trough levels and antibodies in patients with IBD. Inflamm Bowel Dis. 2014;20:2292–8.
Strik AS, van den Brink GR, Ponsioen C, Mathot R, Löwenberg M, D'Haens GR. Suppression of anti-drug antibodies to infliximab or adalimumab with the addition of an immunomodulator in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45:1128–34.
Van Assche G, Magdelaine-Beuzelin C, D'Haens G, et al. Withdrawal of immunosuppression in Crohn’s disease treated with scheduled infliximab maintenance: a randomized trial. Gastroenterology. 2008;134:1861–8.
Louis E, Mary JY, Vernier-Massouille G, et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. 2012;142:63–70 e5; quiz e31.
Casanova MJ, Chaparro M, Domenech E, et al. Safety of thiopurines and anti-TNF-alpha drugs during pregnancy in patients with inflammatory bowel disease. Am J Gastroenterol. 2013;108:433–40.
Akbari M, Shah S, Velayos FS, Mahadevan U, Cheifetz AS. Systematic review and meta-analysis on the effects of thiopurines on birth outcomes from female and male patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:15–22.
Kanis SL, de Lima-Karagiannis A, de Boer NKH, et al. Use of thiopurines during conception and pregnancy is not associated with adverse pregnancy outcomes or health of infants at one year in a prospective study. Clin Gastroenterol Hepatol. 2017;15:1232–1241.e1.
Peyrin-Biroulet L, Oussalah A, Roblin X, Sparrow MP. The use of azathioprine in Crohn’s disease during pregnancy and in the post-operative setting: a worldwide survey of experts. Aliment Pharmacol Ther. 2011;33:707–13.
• Nguyen GC, Seow CH, Maxwell C, et al. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology. 2016;150:734–757.e1 Review of safety of thiopurine use in pregnancy.
Orlando A, Mocciaro F, Renna S, Scimeca D, Rispo A, Lia Scribano M, et al. Early post-operative endoscopic recurrence in Crohn’s disease patients: data from an Italian Group for the study of inflammatory bowel disease (IG-IBD) study on a large prospective multicenter cohort. J Crohns Colitis. 2014;8:1217–21.
• Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: Management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113:481–517 Current United States Society Guideline on treatment of Crohn’s disease including role of thiopurines.
Gionchetti P, Dignass A, Danese S, Magro Dias FJ, Rogler G, Lakatos PL, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 2: surgical management and special situations. J Crohns Colitis. 2017;11:135–49.
Hanauer SB, Korelitz BI, Rutgeerts P, Peppercorn MA, Thisted RA, Cohen RD, et al. Postoperative maintenance of Crohn’s disease remission with 6-mercaptopurine, mesalamine, or placebo: a 2-year trial. Gastroenterology. 2004;127:723–9.
Papay P, Reinisch W, Ho E, Gratzer C, Lissner D, Herkner H, et al. The impact of thiopurines on the risk of surgical recurrence in patients with Crohn’s disease after first intestinal surgery. Am J Gastroenterol. 2010;105:1158–64.
Peyrin-Biroulet L, Deltenre P, Ardizzone S, D'Haens G, Hanauer SB, Herfarth H, et al. Azathioprine and 6-mercaptopurine for the prevention of postoperative recurrence in Crohn’s disease: a meta-analysis. Am J Gastroenterol. 2009;104:2089–96.
Jewel Samadder N, Valentine JF, Guthery S, Singh H, Bernstein CN, Wan Y, et al. Colorectal cancer in inflammatory bowel diseases: a population-based study in Utah. Dig Dis Sci. 2017;62:2126–32.
Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–9.
van Schaik FD, van Oijen MG, Smeets HM, et al. Thiopurines prevent advanced colorectal neoplasia in patients with inflammatory bowel disease. Gut. 2012;61:235–40.
Jess T, Lopez A, Andersson M, et al. Thiopurines and risk of colorectal neoplasia in patients with inflammatory bowel disease: a meta-analysis. Clin Gastroenterol Hepatol. 2014;12:1793–1800.e1.
Zhu Z, Mei Z, Guo Y, Wang G, Wu T, Cui X, et al. Reduced risk of inflammatory bowel disease-associated colorectal neoplasia with use of thiopurines: a systematic review and meta-analysis. J Crohns Colitis. 2018;12:546–58.
Bernheim O, Colombel JF, Ullman TA, Laharie D, Beaugerie L, Itzkowitz SH. The management of immunosuppression in patients with inflammatory bowel disease and cancer. Gut. 2013;62:1523–8.
Shelton E, Laharie D, Scott FI, et al. Cancer recurrence following immune-suppressive therapies in patients with immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology. 2016;151:97–109.e4.
Mehta F. Report: economic implications of inflammatory bowel disease and its management. Am J Manag Care. 2016;22:s51–60.
Kappelman MD, Rifas-Shiman SL, Porter CQ, et al. Direct health care costs of Crohn’s disease and ulcerative colitis in US children and adults. Gastroenterology. 2008;135:1907–13.
Wu N, Lee YC, Shah N, et al. Cost of biologics per treated patient across immune-mediated inflammatory disease indications in a pharmacy benefit management setting: a retrospective cohort study. Clin Ther. 2014;36:1231–41 1241.e1–3.
Sewell JL, Velayos FS. Systematic review: the role of race and socioeconomic factors on IBD healthcare delivery and effectiveness. Inflamm Bowel Dis. 2013;19:627–43.
Yao Q, Altman RD. Thiopurine methyltransferase measurement may not predict azathiopurine-associated non-myelotoxicity. Clin Exp Rheumatol. 2013;31:156.
Chaparro M, Ordas I, Cabre E, et al. Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm Bowel Dis. 2013;19:1404–10.
Godat S, Fournier N, Safroneeva E, Juillerat P, Nydegger A, Straumann A, et al. Frequency and type of drug-related side effects necessitating treatment discontinuation in the Swiss Inflammatory Bowel Disease Cohort. Eur J Gastroenterol Hepatol. 2018;30:612–20.
Teich N, Mohl W, Bokemeyer B, Bündgens B, Büning J, Miehlke S, et al. Azathioprine-induced acute pancreatitis in patients with inflammatory bowel diseases—a prospective study on incidence and severity. J Crohns Colitis. 2016;10:61–8.
Dubinsky MC, Feldman EJ, Abreu MT, Targan SR, Vasiliauskas EA. Thioguanine: a potential alternate thiopurine for IBD patients allergic to 6-mercaptopurine or azathioprine. Am J Gastroenterol. 2003;98:1058–63.
Broekman M, Coenen MJH, Wanten GJ, et al. Risk factors for thiopurine-induced myelosuppression and infections in inflammatory bowel disease patients with a normal TPMT genotype. Aliment Pharmacol Ther. 2017;46:953–63.
de Jong DJ, Goullet M, Naber TH. Side effects of azathioprine in patients with Crohn’s disease. Eur J Gastroenterol Hepatol. 2004;16:207–12.
Gisbert JP, Gomollon F. Thiopurine-induced myelotoxicity in patients with inflammatory bowel disease: a review. Am J Gastroenterol. 2008;103:1783–800.
Kopylov U, Battat R, Benmassaoud A, Paradis-Surprenant L, Seidman EG. Hematologic indices as surrogate markers for monitoring thiopurine therapy in IBD. Dig Dis Sci. 2015;60:478–84.
Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016;48:367–73.
Vogelin M, Biedermann L, Frei P, et al. The impact of azathioprine-associated lymphopenia on the onset of opportunistic infections in patients with inflammatory bowel disease. PLoS One. 2016;11:e0155218.
Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Predictors of infection in rheumatoid arthritis. Arthritis Rheum. 2002;46:2294–300.
Kirchgesner J, Lemaitre M, Carrat F, et al. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology. 2018;155:337–346.e10.
Rahier JF, Ben-Horin S, Chowers Y, Conlon C, de Munter P, D'Haens G, et al. European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2009;3:47–91.
Dayharsh GA, Loftus EV Jr, Sandborn WJ, Tremaine WJ, Zinsmeister AR, Witzig TE, et al. Epstein-Barr virus-positive lymphoma in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine. Gastroenterology. 2002;122:72–7.
•• Lemaitre M, Kirchgesner J, Rudnichi A, et al. Association between use of thiopurines or tumor necrosis factor antagonists alone or in combination and risk of lymphoma in patients with inflammatory bowel disease. JAMA. 2017;318:1679–86 Large database demonstrating small but significant risk of lymphoma with thiopurines alone or in combination with TNF inhibitors.
Kotlyar DS, Lewis JD, Beaugerie L, et al. Risk of lymphoma in patients with inflammatory bowel disease treated with azathioprine and 6-mercaptopurine: a meta-analysis. Clin Gastroenterol Hepatol. 2015;13:847–58.e4 quiz e48–50.
Kotlyar DS, Osterman MT, Diamond RH, Porter D, Blonski WC, Wasik M, et al. A systematic review of factors that contribute to hepatosplenic T-cell lymphoma in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9:36–41 e1.
Parakkal D, Sifuentes H, Semer R, Ehrenpreis ED. Hepatosplenic T-cell lymphoma in patients receiving TNF-alpha inhibitor therapy: expanding the groups at risk. Eur J Gastroenterol Hepatol. 2011;23:1150–6.
Virdis F, Tacci S, Messina F, et al. Hemophagocytic lymphohistiocytosis caused by primary Epstein-Barr virus in patient with Crohn’s disease. World J Gastrointest Surg. 2013;5:306–8.
de Francisco R, Castano-Garcia A, Martinez-Gonzalez S, et al. Impact of Epstein-Barr virus serological status on clinical outcomes in adult patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2018;48:723–30.
Gordon J, Ramaswami A, Beuttler M, Jossen J, Pittman N, Lai J, et al. EBV status and thiopurine use in pediatric IBD. J Pediatr Gastroenterol Nutr. 2016;62:711–4.
Abbas AM, Almukhtar RM, Loftus EV Jr, et al. Risk of melanoma and non-melanoma skin cancer in ulcerative colitis patients treated with thiopurines: a nationwide retrospective cohort. Am J Gastroenterol. 2014;109:1781–93.
• Farraye FA, Melmed GY, Lichtenstein GR, et al. ACG clinical guideline: preventive care in inflammatory bowel disease. Am J Gastroenterol. 2017;112:241–58 Relevance of preventive health maintenance for IBD patients receiving immune suppression.
• Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114:384–413 Current United States Society Guideline on management of ulcerative colitis including role of thiopurines.
Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11:769–84.
Dassopoulos T, Cohen RD, Scherl EJ, Schwartz RM, Kosinski L, Regueiro MD. Ulcerative colitis care pathway. Gastroenterology. 2015;149:238–45.
Gomollon F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3–25.
Sandborn WJ. Crohn’s disease evaluation and treatment: clinical decision tool. Gastroenterology. 2014;147:702–5.
Hanauer SB, Sandborn WJ, Lichtenstein GR. Evolving considerations for thiopurine therapy for inflammatory bowel diseases-a clinical practice update: commentary. Gastroenterology. 2019;156:36–42.
Dassopoulos T, Sultan S, Falck-Ytter YT, et al. American Gastroenterological Association Institute technical review on the use of thiopurines, methotrexate, and anti-TNF-alpha biologic drugs for the induction and maintenance of remission in inflammatory Crohn’s disease. Gastroenterology. 2013;145:1464–78 e1–5.
Hyams JS, Dubinsky M, Rosh J, Ruemmele FM, Eichner SF, Maa JF, et al. The effects of concomitant immunomodulators on the pharmacokinetics, efficacy and safety of adalimumab in paediatric patients with Crohn’s disease: a post hoc analysis. Aliment Pharmacol Ther. 2019;49:155–64.
Colombel JF, Jharap B, Sandborn WJ, Feagan B, Peyrin-Biroulet L, Eichner SF, et al. Effects of concomitant immunomodulators on the pharmacokinetics, efficacy and safety of adalimumab in patients with Crohn’s disease or ulcerative colitis who had failed conventional therapy. Aliment Pharmacol Ther. 2017;45:50–62.
Khanna R, Jairath V, Feagan BG. The evolution of treatment paradigms in Crohn’s disease: beyond better drugs. Gastroenterol Clin N Am. 2017;46:661–77.
Ungaro R, Colombel JF, Lissoos T, Peyrin-Biroulet L. A treat-to-target update in ulcerative colitis: a systematic review. Am J Gastroenterol. 2019 Jun;114(6):874–83.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Saurabh Kapur declares that he has no conflict of interest. Stephen B. Hanauer declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Inflammatory Bowel Disease
Rights and permissions
About this article
Cite this article
Kapur, S., Hanauer, S.B. The Evolving Role of Thiopurines in Inflammatory Bowel Disease. Curr Treat Options Gastro 17, 420–433 (2019). https://doi.org/10.1007/s11938-019-00244-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11938-019-00244-3