Opinion statement
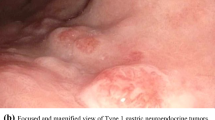
Gastric carcinoid tumors are uncommon, but their percentage among all gastric malignancies has increased to 1.8%. Although they are most often discovered incidentally during endoscopy, gastric carcinoids can present with abdominal pain, bleeding, or symptoms related to the secretion of bioactive substances, most commonly histamine. Gastric carcinoids originate from the foregut and are derived from histamine-containing enterochromaffin-like (ECL) cells. Type I gastric carcinoid, the most common, exhibits slow growth and benign behavior. It occurs within the setting of chronic atrophic gastritis with achlorhydria-induced hypergastrinemia. Gastrin acts directly on ECL cells to induce hyperplasia, dysplasia, and, eventually, neoplasia. Type II gastric carcinoid, the least common type, occurs in patients with gastrinoma-associated multiple endocrine neoplasia syndrome-type 1 (MEN-1). The overall survival is related more to the underlying MEN-1 syndrome than to the gastric carcinoid. Rodents readily develop gastric carcinoid tumors in response to hypergastrinemia. However, in humans, other factors in addition to hypergastrinemia, such as pernicious anemia or MEN-1, must be present, implying that a genetic predisposition is necessary for the development of these tumors. Type III or sporadic gastric carcinoids exhibit a more malignant behavior, with overall 5-year survival rates of less than 50% and normal serum gastrin concentrations. Treatment of all types of gastric carcinoids is predicated upon accurate classification and staging. Radiolabeled somatostatin analogues are superior to conventional radiologic imaging techniques in detecting both primary and metastatic lesions. Treatment of choice for localized disease is excision, either endoscopically or surgically. Antrectomy, by eliminating the trophic effect of gastrin, can be useful for select type I carcinoids. Long-acting somatostatin analogues are excellent palliative agents.
Similar content being viewed by others
References and Recommended Reading
Kidd M, Modlin IM, Mane SM, et al.: Q RT-PCR detection of chromogranin A: a new standard in the identification of neuroendocrine tumor disease. Ann Surg 2006, 243:273–280.
Oberndorfer S: Karzinoide tumoren des dunndarms [in German]. Frankf Z Pathol 1907, 1:237–240.
Godwin JD 2nd: Carcinoid tumors. An analysis of 2,837 cases. Cancer 1975, 36:560–569.
Berge T, Linell F: Carcinoid tumours. Frequency in a defined population during a 12-year period. Acta Pathol Microbiol Scand 1976, 84:322–330.
Hodgson N, Koniaris LG, Livingstone AS, Franceschi D: Gastric carcinoids: a temporal increase with proton pump introduction. Surg Endosc 2005, 19:1610–1612.
Chen D, Zhao CM, Lindstrom E, et al.: Rat stomach ECL cells up-date of biology and physiology. Gen Pharmacol 1999, 32:413–422.
Conlon JM, Deacon CF, Richter G, et al.: Circulating tachykinins (substance P, neurokinin A, neuropeptide K) and the carcinoid flush. Scand J Gastroenterol 1987, 22:97–105.
Solcia E, Rinidi G, Silini E, et al.: Enterochromaffin-like (ECL) cells and their growths: relationship to gastrin, reduced acid secretion and gastritis. Baillieres Clin Gastroenterol 1993, 7:149–165.
Rindi G, Luinetti O, Cornaggia M, et al.: Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology 1993, 104:994–1006.
Manela FD, Ren J, Gao J, et al.: Calcitonin gene-related peptide modulates acid mediated somatostatin and gastrin release from rat antrum. Gastroenterology 1995, 109:701–706.
Galligan CJ, Lawton GP, Tang LH, et al.: Gastric carcinoid tumors: the biology and therapy of an enigmatic and controversial lesion. Am J Gastroenterol 1995, 90:338–352.
Solcia E, Bordi C, Creutzfeld W, et al.: Histopathological classification of nonantral gastric endocrine growths in man. Digestion 1998, 41:185–200.
Bordi C, Yu JY, Baggi MT, et al.: Gastric carcinoids and their precursor lesions. A histologic and immunohistochemical study of 23 cases. Cancer 1991, 67:663–672.
Borch K, Renyall H, Liedberg G, et al.: Relations between circulating gastrin and endocrine cell proliferation in the atrophic gastric fundic mucosa. Scand J Gastroenterol 1986, 21:357–363.
Modlin IM, Lye KD, Kidd M: Carcinoid tumor of the stomach. Surg Oncol 2003, 12:153–172.
Rindi G, Bordi C, Rappel S, et al.: Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J Surg 1996, 20:168–172.
Schindl M, Kaserer K, Niederle B: Treatment of gastric neuroendocrine tumors-the necessity of a type-adapted treatment. Arch Surg 2001, 136:49–54.
Moesta KT, Schlag P: Proposal for a new carcinoid tumor staging system based on tumor tissue infiltration and primary metastasis: a prospective multicentre carcinoid tumor evaluation study. West German Surgical Oncologists’ Group. Eur J Surg Oncol 1990, 16:280–288.
Bordi C, D’Adda T, Azzoni C, Ferraro G: Pathogenesis of ECL cell tumors in humans. Yale J Biol Med 1998, 71:273–284.
Jensen RT: Management of the Zollinger-Ellison syndrome in patients with multiple endocrine neoplasia type 1. J Intern Med 1998, 243:477–488.
Maggard MA, O’Connell JB, Ko CY: Updated population-based review of carcinoid tumors. Ann Surg 2004, 240:117–122.
Bordi C: Endocrine tumors of the stomach. Pathol Res Pract 1995, 191:373–380.
Nobels FR, Kwekkeboom DJ, Coopmans W, et al.: Chromogranin A as serum marker for neuroendocrine neoplasia: comparison with neuron-specific enolase and the alpha-subunit of glycoprotein hormones. J Clin Endocrinol Metab 1997, 82:2622–2628.
Bordi C, Azzoni C, Feraro G, et al.: Sampling strategies for analysis of enterochromaffin-like cell changes in Zollinger-Ellison syndrome. Am J Clin Pathol 2000, 114:419–425.
Giovannini M, Seitz JF, Thomas P, et al.: Endoscopic ultrasound examination using a curved-array transducer in benign and malignant diseases of the stomach. Results in 30 patients [in French]. Gastroenterol Clin Biol 1993, 17:26–32.
Lindstrom E, Hakanson R: Neurohormonal regulation of secretion from isolated rat stomach ECL cells: a critical reappraisal. Regul Pept 2001, 97:169–180.
Modlin IM, Kidd M, Latich I, et al.: Current status of gastrointestinal carcinoids. Gastroenterology 2005, 128:1717–1751.
Lebtahi R, Le Cloirec J, Houzard C, et al.: Detection of neuroendocrine tumors: 99mTc-P829 scintigraphy compared with 111In-pentetreotide scintigraphy. J Nucl Med 2002, 43:889–895.
Gibril F, Reynolds JC, Lubensky IA, et al.: Ability of somatostatin receptor scintigraphy to identify patients with gastric carcinoids: a prospective study. J Nucl Med 2000, 41:1646–1656.
Zuetenhorst JM, Hoefnageli CA, Boot H, et al.: Evaluation of (111)In-pentetreotide, (131)I-MIBG and bone scintigraphy in the detection and clinical management of bone metastases in carcinoid disease. Nucl Med Commun 2002, 23:735–741.
Modlin IM, Lye KD, Kidd M: A 50-year analysis of 562 gastric carcinoids: small tumor or larger problem? Am J Gastroenterol 2004, 99:23–32.
Rappel S, Altendorfhofmann A, Stolte M: Prognosis of gastric carcinoid tumors. Digestion 1995, 56:455–462.
Meko J, Norton J: Management of patients with Zollinger-Ellison syndrome. Ann Rev Med 1995, 46:395–411.
Modlin IM, Kidd M, Lye KD: Biology and management of gastric carcinoid tumors: a review. Eur J Surg 2002, 168:669–683.
Galligan CJ, Lawton GP, Tang LH, et al.: Gastric carcinoid tumors: the biology and therapy of an enigmatic and controversial lesion. Am J Gastroenterol 1995, 90:338–352.
Ichikawa J, Tanabe S, Koizumi W, et al.: Endoscopic mucosal resection in the management of gastric carcinoid tumors. Endoscopy 2003, 35:203–206.
Hirschowitz BI, Griffith J, Pellegrin D, et al.: Rapid regression of enterochromaffinlike cell gastric carcinoids in pernicious anemia after antrectomy. Gastroenterology 1992, 102:1409–1418.
Modlin IM, Latich I, Kidd M, et al.: Therapeutic options for gastrointestinal carcinoids. Clin Gastroenterol Hepatol 2006, 4:526–547.
Welin SV, Janson ET, Sundin A, et al.: High-dose treatment with a long acting somatostatin analogue in patients with disseminated mid-gut carcinoid tumors. Eur J Endocrinol 2004, 151:107–112.
Arnold R, Trautmann ME, Creutzfeld W, et al.: Somatostatin analogue octreotide and inhibition of tumor growth in metastatic endocrine gastroenteropancreatic tumors. Gut 1996, 172:61–67.
Dirix LY, Vermeulen PB, Fierens H, et al.: Long-term results of continuous treatment with recombinant interferon-alpha in patients with metastatic carcinoid tumors—an antiangiogenic effect? Anticancer Drugs 1996, 7:175–181.
Schally A: Oncological applications of somatostatin analogue. Cancer Res 1988, 48:6977–6985.
Hillman N, Herranz L, Alverez C, et al.: Efficacy of octreotide in the regression of a metastatic carcinoid tumor despite negative imaging with In-111-pentetreotide (Octreoscan). Exp Clin Endocrinol Diabetes 1998, 106:226–230.
Kvols LK, Moertel CG, O’Connell MJ, et al.: Treatment of the malignant carcinoid syndrome. Evaluation of a long-acting somatostatin analogue. N Engl J Med 1986, 315:663–666.
Oberg K: Treatment of neuroendocrine tumors. Cancer Treat Rev 1994, 20:331–355.
Oberg K: Future aspects of somatostatin-receptor-mediated therapy. Neuroendocrinology 2004, 80(Suppl 1):57–61.
Frolich JC, Bloomgarden ZT, Oates JA, et al.: The carcinoid flush. Provocation by pentagastrin and inhibition by somatostatin. N Engl J Med 1978, 299:1055–1057.
Dharmsathaphorn K, Sherwin RS, Cataland S, et al.: Somatostatin inhibits diarrhea in the carcinoid syndrome. Ann Intern Med 1980, 92:68–69.
Schnirer II, Yao JC, Ajani JA: Carcinoid—a comprehensive review. Acta Oncol 2003, 42:672–692.
Ahlman H, Kolby L, Lundell, et al.: Clinical management of gastric carcinoid tumors. Digestion 1994, 55Suppl 3):77–85.
Higam AD, Dimaline R, Varro A, et al.: Octreotide suppression test predicts beneficial outcome from antrectomy in a patient with gastric carcinoid tumor. Gastroenterology 1998, 114:817–822.
Dakin GF, Warner RR, Pomp A, et al.: Presentation, treatment, and outcome of type 1 gastric carcinoid tumors. J Surg Oncol 2006, 93:368–372.
Comaru-Schally AM, Schally AV: A clinical overview of carcinoid tumors: perspectives for improvement in treatment using peptide analogs (review). Int J Oncol 2005, 26:301–309.
Kaltsas G, Rockall A, Papadogias D, et al.: Recent advances in radiological and radionuclide imaging and therapy of neuroendocrine tumors. Eur J Endocrinol 2004, 151:15–27.
Krenning EP, Kwekkeboom DJ, Valkema R, et al.: Peptide receptor radionuclide therapy. Ann N Y Acad Sci 2004, 1014:234–245.
Boerlin V, van der Hoek J, Beglinger Ch, et al.: New insights on SOM230, a universal somatostatin receptor ligand. J Endocrinol Invest 2003, 26(8 Suppl):14–16.
Bruns C, Lewis I, Briner U, et al.: SOM230: a novel somatostatin peptidomimetic with broad somatostatin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol 2002, 146:707–716.
Schmid HA, Schoeffter P: Functional activity of the multiligand analog SOM230 at human recombinant somatostatin receptor subtypes supports its usefulness in neuroendocrine tumors. Neuroendocrinology 2004, 80(Suppl 1):47–50.
Kvols L, Oberg K, de Herder W: Early data on the efficacy and safety of the novel multi-ligand somatostatin analog, SOM230, in patients with metastatic carcinoid tumors refractory or resistant to octreotide LAR [abstract]. Proc Am Soc Clin Oncol 2005, 23:8024.
Yao JC, Chamsangavej C, Faria SC: Rapid decrease in blood flow (BF), blood volume (BV), and vascular permeability (PS) in carcinoid patients treated with bevacizumab. ASCO annual meeting proceedings (post-meeting edition) [abstract]. J Clin Oncol 2004, 22:3013.
Carr K, Yao JC, Rashid A: A phase II trial of imatinib in patients with advanced carcinoid tumor. ASCO annual meeting proceedings (post-meeting edition) [abstract]. J Clin Oncol 2004, 22:4124.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hou, W., Schubert, M.L. Treatment of gastric carcinoids. Curr Treat Options Gastro 10, 123–133 (2007). https://doi.org/10.1007/s11938-007-0064-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11938-007-0064-5