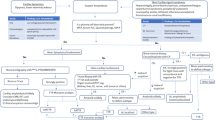
Opinion statement
The systemic amyloidoses are a group of heterogeneous disorders characterized by extracellular deposition of misfolded fibrillar protein that results in organ dysfunction. Involvement of the heart (cardiac amyloidosis) is manifest by increased cardiac wall thickness and impairment of myocardial diastolic and systolic properties, changes that result in heart failure, dysrhythmia, and death. Amyloidosis is classified by precursor protein, with light-chain (AL) and transthyretin (TTR) disease being most common in the United States. TTR amyloid can result from misfolding of variant TTR, a genetically inherited disease, or wild-type TTR, an acquired form of disease (termed senile systemic amyloidosis). In recent years, advances in the diagnosis and treatment of cardiac amyloidosis include identification and validation of disease biomarkers, new imaging techniques, and consensus treatment guidelines. Elevations of B-type natriuretic peptide and cardiac troponins can identify cardiac amyloidosis with a high degree of precision and confer important prognostic information. Non-invasive cardiac imaging techniques, such as cardiac magnetic resonance imaging and echocardiography with strain quantification, afford the ability to diagnose cardiac amyloidosis most often without the need for a confirmatory heart biopsy. Treatment of heart failure resulting from cardiac amyloidosis differs in many respects from most other etiologies of cardiomyopathy. The mainstay of treatment involves volume control with diuretics, low dose β-adrenergic antagonists or amiodarone for dysrhythmia, and warfarin to prevent thromboembolism. Although widely held to have a dismal prognosis, modern treatments such as high-dose melphalan with stem cell transplantation (HDM/SCT) for AL disease achieve a complete hematologic response in nearly half of eligible patients and yield long-term survival. For patients with advanced AL cardiac amyloidosis, cardiac transplantation followed by HDM/SCT is also an option that has proven highly effective. For familial amyloid derived from variant TTR, liver transplantation is the one validated treatment; however, small molecule therapeutic agents now in clinical trials appear capable of slowing or halting TTR amyloid deposition.


Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–96.
Kyle RA, Linos A, Beard CM, et al. Incidence and natural history of primary systemic amyloidosis in Olmsted County, Minnesota, 1950 through 1989. Blood. 1992;79:1817–22.
Connors LH, Lim A, Prokaeva T, et al. Tabulation of human transthyretin (TTR) variants, 2003. Amyloid. 2003;10:160–84.
Jacobson DR, Pastore RD, Yaghoubian R, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med. 1997;336:466–73.
McKinnon, J. The Black Population in the United States: March 2002. US Census Bureau, Washington, D.C. Current Population Reports 2003, Series P20-541.
Buxbaum J, Alexander A, Koziol J, et al. Significance of the amyloidogenic transthyretin Val 122 Ile allele in African Americans in the Arteriosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Am Heart J. 2010;159:864–70.
Connors LH, Prokaeva T, Lim A, et al. Cardiac amyloidosis in African Americans: comparison of clinical and laboratory features of transthyretin V122I amyloidosis and immunoglobulin light chain amyloidosis. Am Heart J. 2009;158:607–14.
Rapezzi C, Quarta CC, Riva L, et al. Transthyretin-related amyloidoses and the heart: a clinical overview. Nat Rev Cardiol. 2010;7:398–408.
Tanskanen M, Peuralinna T, Polvikoski T, et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2- macroglobulin and tau: a population-based autopsy study. Ann Med. 2008;40:232–9.
Dember LM, Hawkins PN, Hazenberg BP, et al. Eprodisate for the treatment of renal disease in AA amyloidosis. N Engl J Med. 2007;356:2349–60.
Murtagh B, Hammill SC, Gertz MA, et al. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol. 2005;95:535–7.
Rapezzi C, Merlini G, Quarta CC, et al. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120:1203–12.
Biolo A, Ramamurthy S, Connors LH, et al. Matrix metalloproteinases and their tissue inhibitors in cardiac amyloidosis: relationship to structural, functional myocardial changes and to light chain amyloid deposition. Circ Heart Fail. 2008;1:249–57.
Brenner DA, Jain M, Pimentel DR, et al. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res. 2004;94:1008–10.
Ng B, Connors LH, Davidoff R, et al. Senile systemic amyloidosis presenting with heart failure: a comparison with light chain-associated amyloidosis. Arch Intern Med. 2005;165:1425–9.
Dispenzieri A, Gertz MA, Kyle RA, et al. Prognostication of survival using cardiac troponins and N-terminal pro-brain natriuretic peptide in patients with primary systemic amyloidosis undergoing peripheral blood stem cell transplantation. Blood. 2004;104:1881–7.
Kristen AV, Giannitsis E, Lehrke S, et al. Assessment of disease severity and outcome in patients with systemic light-chain amyloidosis by the high-sensitivity troponin T assay. Blood. 2010;116:2455–61.
Palladini G, Campana C, Klersy C, et al. Serum N-Terminal pro-brain natriuretic peptide is a sensitive marker of myocardial dysfunction in AL amyloidosis. Circulation. 2003;107:2440–5.
Migrino RQ, Mareedu RK, Eastwood D, et al. Left ventricular ejection time on echocardiography predicts long-term mortality in light chain amyloidosis. J Am Soc Echocardiogr. 2009;22:1396–402.
Koyama J, Falk RH. Prognostic significance of strain Doppler imaging in light-chain amyloidosis. JACC Cardiovasc Imaging. 2010;3:333–42.
Bellavia D, Pellikka PA, Al-Zahrani GB, et al. Independent predictors of survival in primary systemic (AL) Amyloidosis, including cardiac biomarkers and left ventricular strain imaging: an observational cohort study. J Am Soc Echocardiogr. 2010;23:643–52.
Maceira AM, Joshi J, Prasad SK, et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:195–202.
Ruberg FL, Appelbaum E, Davidoff R, et al. Diagnostic and prognostic utility of cardiovascular magnetic resonance imaging in light-chain cardiac amyloidosis. Am J Cardiol. 2009;103:544–9.
Vogelsberg H, Mahrholdt H, Deluigi CC, et al. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis: Noninvasive imaging compared to endomyocardial biopsy. J Am Coll Cardiol. 2008;51:1022–30.
Syed IS, Glockner JF, Feng D, et al. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imaging. 2010;3:155–64.
Austin BA, Tang WH, Rodriguez ER, et al. Delayed hyper-enhancement magnetic resonance imaging provides incremental diagnostic and prognostic utility in suspected cardiac amyloidosis. JACC Cardiovasc Imaging. 2009;2:1369–77.
Maceira A, Prasad S, Hawkins P, et al. Cardiovascular Magnetic Resonance and prognosis in cardiac amyloidosis. J Cardiovasc Magn Reson. 2008;10:54–66.
Glaudemans A, Slart R, Zeebregts C, et al. Nuclear imaging in cardiac amyloidosis. Eur J Nucl Med Mol Imaging. 2009;36:702–14.
Perugini E, Guidalotti PL, Salvi F, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-Diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46:1076–84.
Hongo M, Urushibata K, Kai R, et al. Iodine-123 metaiodobenzylguanidine scintigraphic analysis of myocardial sympathetic innervation in patients with AL (primary) amyloidosis. Am Heart J. 2002;144:122–9.
Hazenberg BP, van Rijswijk MH, Piers DA, et al. Diagnostic performance of 123I-labeled serum amyloid P component scintigraphy in patients with amyloidosis. Am J Med. 2006;119:355.e15–24.
Tsai SB, Seldin DC, Wu H, et al. Myocardial infarction with "clean coronaries" caused by amyloid light-chain AL amyloidosis: a case report and literature review. Amyloid 2011 (in press).
Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. N Engl J Med. 1997;337:898–909.
Dubrey SW, Cha K, Anderson J, et al. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. Q J Med. 1998;91:141–57.
Rubinow A, Skinner M, Cohen AS. Digoxin sensitivity in amyloid cardiomyopathy. Circulation. 1981;63:1285–8.
Gertz MA, Falk RH, Skinner M, et al. Worsening of congestive heart failure in amyloid heart disease treated by calcium channel-blocking agents. Am J Cardiol. 1985;55:1645.
Dhruva SS, Redberg RF. Accelerated approval and possible withdrawal of midodrine. JAMA. 2010;304:2172–3.
Feng D, Edwards WD, Oh JK, et al. Intracardiac thrombosis and embolism in patients with cardiac amyloidosis. Circulation. 2007;116:2420–6.
Feng D, Syed IS, Martinez M, et al. Intracardiac thrombosis and anticoagulation therapy in cardiac amyloidosis. Circulation. 2009;119:2490–7.
Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51.
Sanchorawala V, Skinner M, Quillen K, et al. Long-term outcome of patients with AL amyloidosis treated with high-dose melphalan and stem-cell transplantation. Blood. 2007;110:3561–3.
Jaccard A, Moreau P, Leblond V, et al. High-Dose melphalan versus melphalan plus dexamethasone for AL amyloidosis. N Engl J Med. 2007;357:1083–93.
Dispenzieri A, Lacy MQ, Zeldenrust SR, et al. The activity of lenalidomide with or without dexamethasone in patients with primary systemic amyloidosis. Blood. 2007;109:465–70.
Reece DE, Sanchorawala V, Hegenbart U, et al. Weekly and twice-weekly bortezomib in patients with systemic AL amyloidosis: results of a phase 1 dose-escalation study. Blood. 2009;114:1489–97.
Skinner M, Sanchorawala V, Seldin DC, et al. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann Intern Med. 2004;140:85–93.
Kristen AV, Perz JB, Schonland SO, et al. Rapid progression of left ventricular wall thickness predicts mortality in cardiac light-chain amyloidosis. J Heart Lung Transplant. 2007;26:1313–9.
(Meier-Ewert HK, Sanchorawala V, Berk J, et al. Regression of cardiac wall thickness following chemotherapy and stem cell transplantation for light chain (AL) amyloidosis. Amyloid 2011;18 Suppl 1:125–26.
Kristen AV, Dengler TJ, Hegenbart U, et al. Prophylactic implantation of cardioverter-defibrillator in patients with severe cardiac amyloidosis and high risk for sudden cardiac death. Heart Rhythm. 2008;5:235–40.
Dey BR, Chung SS, Spitzer TR, et al. Cardiac transplantation followed by dose-intensive melphalan and autologous stem-cell transplantation for light chain amyloidosis and heart failure. Transplantation. 2010;90:905–11.
Pilato E, Dell’Amore A, Botta L, et al. Combined heart and liver transplantation for familial amyloidotic neuropathy. Eur J Cardiothorac Surg. 2007;32:180–2.
Hammarstrom P, Wiseman RL, Powers ET, et al. Prevention of transthyretin amyloid disease by changing protein misfolding energetics. Science. 2003;299:713–6.
Disclosure
H.K. Meier-Ewert: none. V. Sanchorawala: none. J.L. Berk is a consultant for Alnylam Pharmaceutical, Isis Pharmaceutical, and Pfizer, and he is supported by NINDS grant NIH R01NS051306. F.L Ruberg has received grants from the American Heart Association (10SDG2550011) and the Amyloidosis Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meier-Ewert, H.K., Sanchorawala, V., Berk, J.L. et al. Cardiac Amyloidosis: Evolving Approach to Diagnosis and Management. Curr Treat Options Cardio Med 13, 528–542 (2011). https://doi.org/10.1007/s11936-011-0147-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11936-011-0147-4