Abstract
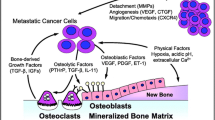
For patients with metastatic prostate cancer, bone is the primary site of tumor localization and the major cause of disease-related morbidity and mortality. Hormonal therapy and chemotherapy alone cannot eradicate disease harbored in bone. The delivery of radiotherapy to the reservoir of disease is an approach previously only achievable using bone-seeking radiopharmaceuticals. Now, however, with the identification of tumor-specific targets, antibodies are being used to deliver radiotherapy to these sites. In this article, we review the rationale behind this approach, the targets being explored, the radiation sources available, and the antibodies currently under clinical development.
Similar content being viewed by others
References and Recommended Reading
Savarese DM, Halabi S, Hars V, et al.: Phase II study of docetaxel, estramustine, and low-dose hydrocortisone in men with hormone-refractory prostate cancer: a final report of CALGB 9780. J Clin Oncol 2001, 19:2509–2516.
Petrylak DP, Macarthur RB, O’Connor J, et al.: Phase I trial of docetaxel with estramustine in androgen-independent prostate cancer. J Clin Oncol 1999, 17:958–967.
Kelly WK, Curley T, Slovin S, et al.: Paclitaxel, estramustine phosphate, and carboplatin in patients with advanced prostate cancer. J Clin Oncol 2001, 19:44–53.
Guise TA, Mundy GR: Cancer and bone. Endocr Rev 1998, 19:18–54.
Scher HI, Chung LWK: Bone metastases: biology and therapy. Semin Oncol 1994, 21:630–656.
Porter AT, McEwan AJB, Powe JE, et al.: Results of a randomized phase III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Radiat Oncol Biol Phys 1993, 25:805–813.
Robinson RG, Preston DF, Schiefelbein M, Baxter KG: Strontium 89 therapy for the palliation of pain due to osseous metastases. JAMA 1995, 274:420–424.
Lewington VJ, McEwan AJ, Ackery DM, et al.: A prospective, randomised double-blind crossover study to examine the efficacy of strontium-89 in pain palliation in patients with advanced prostate cancer metastatic to bone. Eur J Cancer 1991, 27:954–958.
Collins C, Eary JF, Donaldson G, et al.: Samarium-153-EDTMP in bone metastases of hormone refractory prostate carcinoma: a phase I/II trial. J Nucl Med 1993, 34:1839–1844.
Serafini AN, Houston SJ, Resche I, et al.: Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: a double-blind placebo-controlled clinical trial. J Clin Oncol 1998, 16:1574–1581.
van Rensburg AJ, Alberts AS, Louw WK: Quantifying the radiation dosage to individual skeletal lesions treated with samarium-153-EDTMP. J Nucl Med 1998, 39:2110–2115.
Turner SL, Gruenewald S, Spry N, Gebski V: Less pain does equal better quality of life following strontium-89 therapy for metastatic prostate cancer. Br J Cancer 2001, 84:297–302.
Fossa SD, Paus E, Lochoff M, et al.: 89Strontium in bone metastases from hormone resistant prostate cancer: palliation effect and biochemical changes. Br J Cancer 1992, 66:177–180.
Bolger JJ, Dearnaley DP, Kirk D, et al.: Strontium-89 (Metastron) versus external beam radiotherapy in patients with painful bone metastases secondary to prostatic cancer: preliminary report of a multicenter trial. Semin Oncol 1993, 20:32–33.
Tu SM, Millikan RE, Mengistu B, et al.: Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet 2001, 357:326–327. This randomized phase II trial suggested that bone-directed radiation therapy might confer a survival benefit when combined with chemotherapy. A phase III trial testing this strategy is underway.
Taplin ME, Bubley GJ, Shuster TD, et al.: Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med 1995, 332:1393–1398.
Koivisto P, Visakorpi T, Kallioniemi OP: Androgen receptor gene amplification: a novel molecular mechanism for endocrine therapy resistance in human prostate cancer. Scand J Clin Lab Invest 1996, 226:57–63.
Osman I, Scher HI, Drobnjak M, et al.: HER-2/neu (p185neu) protein expression in the natural and treated history of prostate cancer. Clin Cancer Res 2001, 7:2643–2647.
McDonnell TJ, Troncoso P, Brisbay SM, et al.: Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res 1992, 52:6940–6944.
Ow KT, Mameghan D, Lochhead A, et al.: The prognostic significance of tumor-associated markers p53, HER-2/neu, c-myc, v-Hras, PCNA and EGFr in local and distant recurrence in localized human prostatic adenocarcinoma. Urol Oncol 1995, 144:152.
Scher HI, Heller G: Clinical states in prostate cancer: towards a dynamic model of disease progression. Urology 2000, 55:323–327.
Morris MJ, Reuter VE, Kelly WK, et al.: HER-2 profiling and targeting in prostate carcinoma. Cancer 2002, 94:980–986.
Reiter RE, Gu Z, Watabe T, et al.: Prostate stem cell antigen: a cell surface marker overexpressed in prostate cancer. Proc Natl Acad Sci U S A 1998, 95:1735–1740.
Gu Z, Thomas G, Yamashiro J, et al.: Prostate stem cell antigen (PSCA) expression increases with high Gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene 2000, 19:1288–1296.
Israeli RS, Powell CT, Fair WR, Heston WD: Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res 1993, 53:227–230.
Wright J, WL, Haley C, Beckett ML, Schellhammer PF: Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol 1995, 1:18–28.
Wright GL Jr, Grob BM, Haley C, et al.: Upregulation of prostate-specific membrane antigen after androgendeprivation therapy. Urology 1996, 48:326–334.
Smith-Jones PM, Vallabahajosula S, Goldsmith SJ, et al.: In vitro characterization of radiolabeled monoclonal antibodies specific for the extracellular domain of prostate-specific membrane antigen. Cancer Res 2000, 60:5237–5243. This comparison of multiple antibodies targeting PSMA illustrates how the properties of the antibody—from binding site, to affinity, to the rate of internalization—can be used to select the one most appropriate for radioimmunotherapy.
Chang SS, Reuter VE, Heston WD, et al.: Five different antiprostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res 1999, 59:3192–3198.
Thor A, Ohuchi N, Szpak CA, et al.: The distribution of onocfetal antigen TAG-72 defined by monoclonal antibody B73.2. Cancer Res 1986, 46:3118–3124.
Gallinger S, Reilly RM, Kirsh JG, et al.: Comparative dual label study of first and second generation antitumor-associated glycoprotein-72 monoclonal antibodies in colorectal cancer patients. Cancer Res 1993, 53:271–278.
Myers RB, Meredith RF, Schlom J, et al.: Tumor associated glycoprotein-72 is highly expressed in prostatic adenocarcinomas. J Urol 1994, 152:243–246.
Brenner PC, Rettig WJ, Sanz-Moncasi MP, et al.: TAG-72 expression in primary, metastatic and hormonally treated prostate cancer as defined by monoclonal antibody CC49. J Urol 1995, 153:1575–1579.
Babaian RJ, Murray JL, Lamki LM, et al.: Radioimmunological imaging of metastatic prostatic cancer with 111indium-labeled monoclonal antibody PAY 276. J Urol 1987, 137:439–443.
Halpern SE, Haindl W, Beauregard J, et al.: Scintigraphy with In-111-labeled monoclonal antitumor antibodies: kinetics, biodistribution, and tumor detection. Radiology 1988, 168:529–536.
Colcher D, Minelli MF, Roselli M, et al.: Radioimmunolocalization of human carcinoma xenografts with B72.3 second generation monoclonal antibodies. Cancer Res 1988, 48:4597–4603.
Divgi CR, Scott AM, Dantis L, et al.: Phase I radioimmunotherapy trial with iodine-131-CC49 in metastatic colon carcinoma. J Nucl Med 1995, 36:586–592.
Meredith RF, Bueschen AJ, Khazaeli MB, et al.: Treatment of metastatic prostate carcinoma with radiolabeled antibody CC49. J Nucl Med 1994, 35:1017–1022.
Slovin SF, Scher HI, Divgi CR, et al.: Interferon-gamma and monoclonal antibody 131I-labeled CC49: outcomes in patients with androgen-independent prostate cancer. Clin Cancer Res 1998, 4:643–651. This study illustrates the concept of target modulation as a means of maximizing the effects of RIT.
Deb N, Goris M, Trisler K, et al.: Treatment of hormonerefractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clin Cancer Res 1996, 2:1289–1297.
Liu H, Moy P, Kim S, et al.: Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res 1997, 57:3629–3634.
Smith-Jones PM, Vallabhajosula S, Navarro V, et al.: Radiolabeled monoclonal antibodies specific to the extracellular domain of prostate-specific membrane antigen: preclinical studies in nude mice bearing LNCaP human prostate tumor. J Nucl Med 2003, 44:610–617.
Morris MJ, Pandit-Taskar N, Divgi C, et al.: Pilot trial of anti-PSMA antibody J591 for prostate cancer [abstract]. Proc ASCO 2003, 22:407.
Milowsky MI, Joyce M, Berger F, et al.: Phase I trial results of yttrium-90 (90Y)-labeled anti-prostate specific membrane antigen (PSMA) monoclonal antibody (mAb) J591 in the treatment of patients with advanced prostate cancer [abstract]. Proc ASCO 2003, 22:394.
Bander NH, Trabulsi EJ, Kostakoglu L, et al.: Targeting metastatic prostate cancer with radiolabeled monoclonal antibody J591 to the extracellular domain of prostate specific membrane antigen. J Urol 2003, 170:1717–1721. This paper presented the initial targeting data regarding J591, an antibody targeting the external domain of PSMA that can be repetitively dosed.
Bander NH, Nanus DM, Milowsky MI, et al.: Phase I radioimmunotherapy (RIT) trial of humanized monoclonal (mAb) antibody J591 to the extracellular domain of prostate specific membrane antigen (PSMAext) radiolabeled with 177leutetium (177Lu) in advanced prostate cancer (Pca) [abstract]. Proc ASCO 2003, 22:401.
Bander NH, Nanus DM, Milowsky MI, et al.: Targeted systemic therapy of prostate cancer with a monoclonal antibody to prostate-specific membrane antigen. Semin Oncol 2003, 30:667–676.
McDevitt MR, Barendswaard E, Ma D, et al.: An alpha-particle emitting antibody ([213Bi]J591) for radioimmunotherapy of prostate cancer. Cancer Res 2000, 60:6095–6100.
Ross S, Spencer SD, Holcomb I, et al.: Prostate stem cell antigen as therapy target: tissue expression and in vivo efficacy of an immunoconjugate. Cancer Res 2002, 62:2546–2553.
Rydh A, Riklund Ahlstrom K, Widmark A, et al.: Radioimmunoscintigraphy with a novel monoclonal antiprostate antibody (E4): an experimental study in nude mice. Cancer 1997, 80:2398–2403.
O’Donnell RT, DeNardo SJ, Yuan A, et al.: Radioimmunotherapy with (111)In/(90)Y-2IT-BAD-m170 for metastatic prostate cancer. Clin Cancer Res 2001, 7:1561–1568.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Morris, M.J., Pandit-Taskar, N., Divgi, C. et al. Targeting osseous metastases: rationale and development of radioimmunotherapy for prostate cancer. Curr Urol Rep 6, 163–170 (2005). https://doi.org/10.1007/s11934-005-0003-8
Issue Date:
DOI: https://doi.org/10.1007/s11934-005-0003-8