Abstract
Purpose of the Review
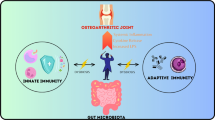
The microbiome has recently emerged as a powerful contributor to health and illness in chronic, systemic disorders. Furthermore, new microbiome niches beyond traditional gut locations are frequently being described. Over the past 5 years, numerous pivotal studies have demonstrated associations between changes in various microbiome niches and the development of osteoarthritis (OA). Herein, we review the most impactful recent literature, including microbiome associations with disease and the potential therapeutic value of microbiome manipulation.
Recent Findings
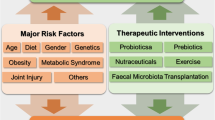
The gut microbiome of human OA patients is enriched in specific bacterial clades, most notably Streptococcus, which correlates with OA pain, Firmicutes, and others. Most studies have focused on knee OA, although one publication demonstrated positive associations with 3 gut microbiome clades in hand OA. OA can be easily distinguished from RA by evaluating differences in oral microbiome composition. Most studies have also demonstrated a reduction in richness of the gut microbiome (alpha diversity) associated with OA. Several studies have identified bacterial signatures within human knee and hip cartilage, synovial fluid, and synovial tissue and have described changes in these patterns occurring with the development of OA. In animal models of OA, high-fat diet-induced obesity has been the most well-studied OA risk factor associated with changes in the microbiome, with numerous bacterial clades changed within the gut microbiome and associated with OA. Also in animal models, various oral supplementations, including dietary fiber, probiotics including Lactobacillus species, and cecal microbiome transplantation have all shown improvements in OA histopathology or cartilage healing.
Summary
Microbiome changes are strongly associated with the OA disease process and with individual OA risk factors related to both the gut microbiome and the microbial DNA patterns in the joint. Microbiome-directed interventions have the potential to prevent or reduce the progression of OA. Future studies should investigate the mechanistic underpinnings of these microbiome associations and further define the therapeutic potential of microbiome augmentation.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. Pain Ovid Technologies (Wolters Kluwer Health). 2010;149:573–81.
McAlindon TE, Cooper C, Kirwan JR, Dieppe PA. Determinants of disability in osteoarthritis of the knee. Ann Rheum Dis BMJ. 1993;52:258–62.
Sharif B, Garner R, Sanmartin C, Flanagan WM, Hennessy D, Marshall DA. Risk of work loss due to illness or disability in patients with osteoarthritis: a population-based cohort study. Rheumatology (Oxford). Oxford University Press (OUP); 2016;55:861–8.
Gleicher Y, Croxford R, Hochman J, Hawker G. A prospective study of mental health care for comorbid depressed mood in older adults with painful osteoarthritis. BMC Psychiatry. Springer Science and Business Media LLC; 2011;11:147.
Veronese N, Cereda E, Maggi S, Luchini C, Solmi M, Smith T, et al. Osteoarthritis and mortality: a prospective cohort study and systematic review with meta-analysis. Semin Arthritis Rheum Elsevier BV. 2016;46:160–7.
McDonough CM, Jette AM. The contribution of osteoarthritis to functional limitations and disability. Clin Geriatr Med Elsevier BV. 2010;26:387–99.
Centers for Disease Control and Prevention (CDC). Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation --- United States, 2007–2009. MMWR Morb Mortal Wkly Rep. 2010;59:1261–5.
Arthritis-related statistics | Data and Statistics | Arthritis | CDC [Internet]. 2018 [cited 2018 Jan 31]. Available from: https://www.cdc.gov/arthritis/data_statistics/arthritis-related-stats.htm. Accessed 15 Jan 2022.
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96.
Centers for Disease Control and Prevention (CDC). Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation--United States, 2010–2012. MMWR Morb Mortal Wkly Rep. 2013;62:869–73.
Yelin E, Murphy L, Cisternas MG, Foreman AJ, Pasta DJ, Helmick CG. Medical care expenditures and earnings losses among persons with arthritis and other rheumatic conditions in 2003, and comparisons with 1997. Arthritis Rheum. 2007;56:1397–407.
Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94:201–7.
Wallace IJ, Worthington S, Felson DT, Jurmain RD, Wren KT, Maijanen H, et al. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proceedings of the National Academy of Sciences [Internet]. 2017; Available from: http://www.pnas.org/content/early/2017/08/08/1703856114.abstract. Accessed 15 Jan 2022.
Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26.
Sandell LJ. Etiology of osteoarthritis: genetics and synovial joint development. Nat Rev Rheumatol. 2012;8:77–89.
Magnusson K, Scurrah K, Ystrom E, Ørstavik RE, Nilsen T, Steingrímsdóttir ÓA, et al. Genetic factors contribute more to hip than knee surgery due to osteoarthritis - a population-based twin registry study of joint arthroplasty. Osteoarthritis Cartilage. 2017;25:878–84.
Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr Opin Rheumatol. 2008;20:565–72.
Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533.
Matijašić M, Meštrović T, Paljetak HČ, Perić M, Barešić A, Verbanac D. Gut microbiota beyond bacteria-mycobiome, virome, archaeome, and eukaryotic parasites in IBD. Int J Mol Sci. MDPI AG; 2020;21:2668.
Petrova P, Petrov K. Prebiotic–probiotic relationship: the genetic fundamentals of polysaccharides conversion by bifidobacterium and lactobacillus genera. Food Bioconversion. Elsevier; 2017. p. 237–78.
Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol Elsevier BV. 2011;9:1044–9.
Johnson JS, Spakowicz DJ, Hong B-Y, Petersen LM, Demkowicz P, Chen L, et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat Commun. 2019;10:5029.
DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. American Society for Microbiology; 2006;72:5069–72.
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res Oxford University Press (OUP). 2013;41:D590–6.
Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, et al. Ribosomal database project: data and tools for high throughput rRNA analysis. Nucleic Acids Res Oxford University Press (OUP). 2014;42:D633–42.
Benson DA, Cavanaugh M, Clark K, Karsch-Mizrachi I, Ostell J, Pruitt KD, et al. GenBank Nucleic acids Res. 2018;46:D41–7.
Dunn CM, Velasco C, Rivas A, Andrews M, Garman C, Jacob PB, et al. Identification of cartilage microbial DNA signatures and associations with knee and hip osteoarthritis. Arthritis rheumatol Wiley. 2020;72:1111–22. First demonstration of cartilage microbial DNA signatures.
Témoin S, Chakaki A, Askari A, El-Halaby A, Fitzgerald S, Marcus RE, et al. Identification of oral bacterial DNA in synovial fluid of patients with arthritis with native and failed prosthetic joints. J Clin Rheumatol. 2012;18:117–21.
Tsai JC, Casteneda G, Lee A, Dereschuk K, Li WT, Chakladar J, et al. Identification and characterization of the intra-articular microbiome in the osteoarthritic knee. Int J Mol Sci [Internet]. 2020;21. Available from: https://doi.org/10.3390/ijms21228618. First demonstration of synovium microbial DNA signatures in OA.
Zhao Y, Chen B, Li S, Yang L, Zhu D, Wang Y, et al. Detection and characterization of bacterial nucleic acids in culture-negative synovial tissue and fluid samples from rheumatoid arthritis or osteoarthritis patients. Sci Rep. 2018;8:14305.
Amarasinghe SL, Su S, Dong X, Zappia L, Ritchie ME, Gouil Q. Opportunities and challenges in long-read sequencing data analysis. Genome Biol. Springer Science and Business Media LLC; 2020;21:30.
Ranjan R, Rani A, Metwally A, McGee HS, Perkins DL. Analysis of the microbiome: advantages of whole genome shotgun versus 16S amplicon sequencing. Biochem Biophys Res Commun Elsevier BV. 2016;469:967–77.
Boer CG, Radjabzadeh D, Uitterlinden AG, Kraaij R, van Meurs JB. The role of the gut microbiome in osteoarthritis and joint pain. Osteoarthritis Cartilage. Elsevier; 2017;25:S10. The first, and still largest, study investigating fecal microbiome changes linked with human OA.
Kho ZY, Lal SK. The human gut microbiome - a potential controller of wellness and disease. Front Microbiol. 2018;9:1835.
Tap J, Mondot S, Levenez F, Pelletier E, Caron C, Furet J-P, et al. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol Wiley. 2009;11:2574–84.
Dreyer JL, Liebl AL. Early colonization of the gut microbiome and its relationship with obesity. Hum microbiome j Elsevier BV. 2018;10:1–5.
Wilmanski T, Diener C, Rappaport N, Patwardhan S, Wiedrick J, Lapidus J, et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat Metab. Springer Science and Business Media LLC; 2021;3:274–86.
Mancabelli L, Milani C, Lugli GA, Turroni F, Ferrario C, van Sinderen D, et al. Meta-analysis of the human gut microbiome from urbanized and pre-agricultural populations. Environ Microbiol Wiley. 2017;19:1379–90.
Sonnenburg ED, Sonnenburg JL. The ancestral and industrialized gut microbiota and implications for human health. Nat Rev Microbiol. 2019;17:383–90.
Obregon-Tito AJ, Tito RY, Metcalf J, Sankaranarayanan K, Clemente JC, Ursell LK, et al. Subsistence strategies in traditional societies distinguish gut microbiomes. Nat Commun. Springer Science and Business Media LLC; 2015;6:6505.
Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. Springer Science and Business Media LLC; 2012;486:222–7.
Wei J, Zhang C, Zhang Y, Zhang W, Doherty M, Yang T, et al. Association between gut microbiota and symptomatic hand osteoarthritis: data from the Xiangya osteoarthritis study. Arthritis Rheumatol. 2021;73:1656–62.
Chen J, Wang A, Wang Q. Dysbiosis of the gut microbiome is a risk factor for osteoarthritis in older female adults: a case control study. BMC Bioinformatics. 2021;22:299.
Rushing BR, McRitchie S, Arbeeva L, Nelson AE, Azcarate-Peril MA, Li Y-Y, et al. Fecal metabolomics reveals products of dysregulated proteolysis and altered microbial metabolism in obesity-related osteoarthritis. Osteoarthritis Cartilage [Internet]. 2021; Available from: https://doi.org/10.1016/j.joca.2021.10.006
Wang Z, Zhu H, Jiang Q, Zhu YZ. The gut microbiome as non-invasive biomarkers for identifying overweight people at risk for osteoarthritis. Microb Pathog. 2021;157:104976.
Chen B, Zhao Y, Li S, Yang L, Wang H, Wang T, et al. Variations in oral microbiome profiles in rheumatoid arthritis and osteoarthritis with potential biomarkers for arthritis screening. Sci Rep. 2018;8:17126.
Lei M, Guo C, Wang D, Zhang C, Hua L. The effect of probiotic Lactobacillus casei Shirota on knee osteoarthritis: a randomised double-blind, placebo-controlled clinical trial. Benef Microbes. 2017;8:697–703. First demonstration of a potential pain benefit to knee OA patients from taking a specific-strain probiotic.
Daïen CI, Pinget GV, Tan JK, Macia L. Detrimental impact of microbiota-accessible carbohydrate-deprived diet on gut and immune homeostasis: an overview. Front Immunol. Frontiers Media SA; 2017;8:548.
Favazzo LJ, Hendesi H, Villani DA, Soniwala S, Dar Q-A, Schott EM, et al. The gut microbiome-joint connection: implications in osteoarthritis. Curr Opin Rheumatol. 2020;32:92–101.
Huang Z, Chen J, Li B, Zeng B, Chou C-H, Zheng X, et al. Faecal microbiota transplantation from metabolically compromised human donors accelerates osteoarthritis in mice. Ann Rheum Dis. 2020;79:646–56. First demonstration of a direct link between human metabolic syndrome-microbiome changes and OA in mice.
Ulici V, Kelley KL, Azcarate-Peril MA, Cleveland RJ, Sartor RB, Schwartz TA, et al. Osteoarthritis induced by destabilization of the medial meniscus is reduced in germ-free mice. Osteoarthritis Cartilage. 2018;26:1098–109. Pivotal study showing reduced OA pathology following DMM surgery in germ-free mice.
Miotla Zarebska J, Pearson C, Selby J, Moussa C, Scott B, dos Santos Duarte C, et al. The microbiome does not influence cartilage degradation following joint destabilisation in mice when performed under stringently controlled conditions. Osteoarthritis Cartilage. Elsevier; 2021;29:S190.
Guan Z, Jia J, Zhang C, Sun T, Zhang W, Yuan W, et al. Gut microbiome dysbiosis alleviates the progression of osteoarthritis in mice. Clin Sci [Internet]. 2020; Available from: https://doi.org/10.1042/CS20201224
Mendez ME, Murugesh DK, Sebastian A. Antibiotic treatment prior to injury improves post-traumatic osteoarthritis outcomes in mice. International journal of [Internet]. mdpi.com; 2020; Available from: https://www.mdpi.com/1422-0067/21/17/6424
Schott EM, Farnsworth CW, Grier A, Lillis JA, Soniwala S, Dadourian GH, et al. Targeting the gut microbiome to treat the osteoarthritis of obesity. JCI Insight [Internet]. 2018;3. Available from: https://doi.org/10.1172/jci.insight.95997
Griffin TM, Huebner JL, Kraus VB, Yan Z, Guilak F. Induction of osteoarthritis and metabolic inflammation by a very high fat diet in mice: effects of short-term exercise. Arthritis Rheum. NIH Public Access; 2012;64:443.
Guss JD, Ziemian SN, Luna M, Sandoval TN, Holyoak DT, Guisado GG, et al. The effects of metabolic syndrome, obesity, and the gut microbiome on load-induced osteoarthritis. Osteoarthritis Cartilage. 2019;27:129–39.
Loeser RF, Arbeeva L, Kelley K, Fodor AA, Sun S, Ulici V, et al. Association of Increased serum lipopolysaccharide but not microbial dysbiosis with obesity-related osteoarthritis. Arthritis Rheumatol [Internet]. 2021; Available from: https://doi.org/10.1002/art.41955
Huang Z, Stabler T, Pei F, Kraus V. Both systemic and local lipopolysaccharide (LPS) burden is associated with knee osteoarthritis (OA). Osteoarthritis Cartilage Elsevier. 2016;24:S329–30. Important study linking increases in bacterial wall proteins (LPS) with human knee OA.
Collins KH, Schwartz DJ, Lenz KL, Harris CA, Guilak F. Taxonomic changes in the gut microbiota are associated with cartilage damage independent of adiposity, high fat diet, and joint injury. Sci Rep. 2021;11:14560.
Collins KH, Lenz KL, Pollitt EN, Ferguson D, Hutson I, Springer LE, et al. Adipose tissue is a critical regulator of osteoarthritis. Proc Natl Acad Sci U S A [Internet]. 2021;118. Available from: https://doi.org/10.1073/pnas.2021096118
Velasco C, Dunn C, Sturdy C, Izda V, Martin J, Rivas A, et al. Ear wound healing in MRL/MpJ mice is associated with gut microbiome composition and is transferable to non-healer mice via microbiome transplantation. PLoS One. 2021;16:e0248322.First demonstration of microbiome transplantation as a potential therapeutic for earhole cartilage healing, a trait closely linked with OA protection.
Rai MF, Hashimoto S, Johnson EE, Janiszak KL, Fitzgerald J, Heber-Katz E, et al. Heritability of articular cartilage regeneration and its association with ear wound healing in mice. Arthritis Rheum. 2012;64:2300–10.
Ma H-L, Blanchet TJ, Peluso D, Hopkins B, Morris EA, Glasson SS. Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthritis Cartilage. 2007;15:695–700.
Song W, Liu Y, Dong X, Song C, Bai Y, Hu P, et al. Lactobacillus M5 prevents osteoarthritis induced by a high-fat diet in mice. J Funct Foods. Elsevier BV; 2020;72:104039.
Lee SH, Kwon JY, Jhun J, Jung K, Park S-H, Yang CW, et al. Lactobacillus acidophilus ameliorates pain and cartilage degradation in experimental osteoarthritis. Immunol Lett. 2018;203:6–14.
Jhun J, Cho K-H, Lee D-H, Kwon JY, Woo JS, Kim J, et al. Oral administration of Lactobacillus rhamnosus ameliorates the progression of osteoarthritis by inhibiting joint pain and inflammation. Cells [Internet]. 2021;10. Available from: https://doi.org/10.3390/cells10051057
Kwon JY, Lee SH, Jhun J, Choi J, Jung K, Cho KH, et al. The combination of probiotic complex, rosavin, and zinc improves pain and cartilage destruction in an osteoarthritis rat model. J Med Food. 2018;21:364–71.
Rios JL, Hart DA, Reimer RA, Herzog W. Prebiotic and exercise do not alter knee osteoarthritis in a rat model of established obesity. Cartilage. 2021;13:1456S-1466S.
Wallace IJ, Bendele AM, Riew G, Frank EH, Hung H-H, Holowka NB, et al. Physical inactivity and knee osteoarthritis in guinea pigs. Osteoarthritis Cartilage. 2019;27:1721–8.
Henrotin Y, Patrier S, Pralus A, Roche M, Nivoliez A. Protective actions of oral administration of Bifidobacterium longum CBi0703 in spontaneous osteoarthritis in Dunkin Hartley guinea pig model. Cartilage. 2021;13:1204S-1213S.
Cintio M, Scarsella E, Sgorlon S, Sandri M, Stefanon B. Gut microbiome of healthy and arthritic dogs. Vet Sci. MDPI AG; 2020;7:92.
Hahn AK, Wallace CW, Welhaven HD, Brooks E, McAlpine M, Christiansen BA, et al. The microbiome mediates epiphyseal bone loss and metabolomic changes after acute joint trauma in mice. Osteoarthritis Cartilage. 2021;29:882–93.
Funding
This work was supported by NIH grants K08AR070891, P20GM125528, R61AR078075, and R01AR076440, and the Department of Defense CDMRP grant PR191652. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding source was not involved in the writing of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Osteoarthritis
Rights and permissions
About this article
Cite this article
Dunn, C.M., Jeffries, M.A. The Microbiome in Osteoarthritis: a Narrative Review of Recent Human and Animal Model Literature. Curr Rheumatol Rep 24, 139–148 (2022). https://doi.org/10.1007/s11926-022-01066-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-022-01066-6