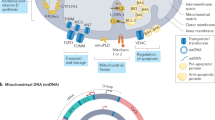
Abstract
Purpose of Review
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by autoantibody production and inflammation in multiple organs. In this article, we present data on how various mitochondria pathologies are involved in the pathogenesis of the disease including the fact that they serve as a reservoir of autoantigens which contribute to the upending of lymphocyte tolerance.
Recent Findings
Mitochondrial DNA from various cell sources, including neutrophil extracellular traps, platelets, and red blood cells, elicits the production of type I interferon which contributes to breaking of peripheral tolerance. Mitochondrial DNA also serves as autoantigen targeted by autoantibodies. Mutations of mitochondrial DNA triggered by reactive oxygen species induce T cell cross-reactivity against self-antigens. Selective gene polymorphisms that regulate mitochondrial apoptosis in autoreactive B and T cells represent another key aspect in the induction of autoimmunity.
Summary
Various mitochondrial abnormalities, including changes in mitochondrial function, oxidative stress, genetic polymorphism, mitochondrial DNA mutations, and apoptosis pathways, are each linked to different aspects of lupus pathogenesis. However, whether targeting these mitochondrial pathologies can be used to harness autoimmunity remains to be explored.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Abrahams JP, Leslie AG, Lutter R, Walker JE. Structure at 2.8 A resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370(6491):621–8. https://doi.org/10.1038/370621a0.
Suen DF, Narendra DP, Tanaka A, Manfredi G, Youle RJ. Parkin overexpression selects against a deleterious mtDNA mutation in heteroplasmic cybrid cells. Proc Natl Acad Sci U S A. 2010;107(26):11835–40. https://doi.org/10.1073/pnas.0914569107.
Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365(22):2110–21. https://doi.org/10.1056/NEJMra1100359.
Tsokos GC. Autoimmunity and organ damage in systemic lupus erythematosus. Nat Immunol. 2020;21(6):605–14. https://doi.org/10.1038/s41590-020-0677-6.
Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201(5):703–11. https://doi.org/10.1084/jem.20042251.
Guo W, Smith D, Aviszus K, Detanico T, Heiser RA, Wysocki LJ. Somatic hypermutation as a generator of antinuclear antibodies in a murine model of systemic autoimmunity. J Exp Med. 2010;207(10):2225–37. https://doi.org/10.1084/jem.20092712.
Wellmann U, Letz M, Herrmann M, Angermüller S, Kalden JR, Winkler TH. The evolution of human anti-double-stranded DNA autoantibodies. Proc Natl Acad Sci U S A. 2005;102(26):9258–63. https://doi.org/10.1073/pnas.0500132102.
Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206(3):561–76. https://doi.org/10.1084/jem.20081886.
Lang BF, Burger G, O’Kelly CJ, Cedergren R, Golding GB, Lemieux C, et al. An ancestral mitochondrial DNA resembling a eubacterial genome in miniature. Nature. 1997;387(6632):493–7. https://doi.org/10.1038/387493a0.
Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–7. https://doi.org/10.1038/nature08780.
Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290(5806):457–65. https://doi.org/10.1038/290457a0.
Vyshkina T, Sylvester A, Sadiq S, Bonilla E, Canter JA, Perl A, et al. Association of common mitochondrial DNA variants with multiple sclerosis and systemic lupus erythematosus. Clin Immunol. 2008;129(1):31–5. https://doi.org/10.1016/j.clim.2008.07.011.
Jönsen A, Yu X, Truedsson L, Nived O, Sturfelt G, Ibrahim S, et al. Mitochondrial DNA polymorphisms are associated with susceptibility and phenotype of systemic lupus erythematosus. Lupus. 2009;18(4):309–12. https://doi.org/10.1177/0961203308097477.
Tang Y, Wang L, Zhu M, Yang M, Zhong K, Du Q, et al. Association of mtDNA M/N haplogroups with systemic lupus erythematosus: a case-control study of Han Chinese women. Sci Rep. 2015;5:10817. https://doi.org/10.1038/srep10817.
Lee HT, Lin CS, Chen WS, Liao HT, Tsai CY, Wei YH. Leukocyte mitochondrial DNA alteration in systemic lupus erythematosus and its relevance to the susceptibility to lupus nephritis. Int J Mol Sci. 2012;13(7):8853–68. https://doi.org/10.3390/ijms13078853.
Yu X, Wester-Rosenlöf L, Gimsa U, Holzhueter SA, Marques A, Jonas L, et al. The mtDNA nt7778 G/T polymorphism affects autoimmune diseases and reproductive performance in the mouse. Hum Mol Genet. 2009;18(24):4689–98. https://doi.org/10.1093/hmg/ddp432.
Alam K, Moinuddin, Jabeen S. Immunogenicity of mitochondrial DNA modified by hydroxyl radical. Cell Immunol. 2007;247(1):12–7. https://doi.org/10.1016/j.cellimm.2007.06.007.
Parsons TJ, Muniec DS, Sullivan K, Woodyatt N, Alliston-Greiner R, Wilson MR, et al. A high observed substitution rate in the human mitochondrial DNA control region. Nat Genet. 1997;15(4):363–8. https://doi.org/10.1038/ng0497-363.
Chen L, Duvvuri B, Grigull J, Jamnik R, Wither JE, Wu GE. Experimental evidence that mutated-self peptides derived from mitochondrial DNA somatic mutations have the potential to trigger autoimmunity. Hum Immunol. 2014;75(8):873–9. https://doi.org/10.1016/j.humimm.2014.06.012.
López-López L, Nieves-Plaza M, Castro Mdel R, Font YM, Torres-Ramos CA, Vilá LM, et al. Mitochondrial DNA damage is associated with damage accrual and disease duration in patients with systemic lupus erythematosus. Lupus. 2014;23(11):1133–41. https://doi.org/10.1177/0961203314537697.
Lee HM, Sugino H, Aoki C, Nishimoto N. Underexpression of mitochondrial-DNA encoded ATP synthesis-related genes and DNA repair genes in systemic lupus erythematosus. Arthritis Res Ther. 2011;13(2):R63. https://doi.org/10.1186/ar3317.
Al-Shobaili HA, Rasheed Z. Physicochemical and immunological studies on mitochondrial DNA modified by peroxynitrite: implications of neo-epitopes of mitochondrial DNA in the etiopathogenesis of systemic lupus erythematosus. Lupus. 2013;22(10):1024–37. https://doi.org/10.1177/0961203313498803.
Caielli S, Athale S, Domic B, Murat E, Chandra M, Banchereau R, et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J Exp Med. 2016;213(5):697–713. https://doi.org/10.1084/jem.20151876.
Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, De Ravin SS, Smith CK, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22(2):146–53. https://doi.org/10.1038/nm.4027.
Wang H, Li T, Chen S, Gu Y, Ye S. Neutrophil extracellular trap mitochondrial DNA and its autoantibody in systemic lupus erythematosus and a proof-of-concept trial of metformin. Arthritis Rheumatol. 2015;67(12):3190–200. https://doi.org/10.1002/art.39296.
Blanco LP, Pedersen HL, Wang X, Lightfoot YL, Seto N, Carmona-Rivera C, et al. Improved mitochondrial metabolism and reduced inflammation following attenuation of murine lupus with coenzyme Q10 analog idebenone. Arthritis Rheumatol. 2020;72(3):454–64. https://doi.org/10.1002/art.41128.
Melki I, Allaeys I, Tessandier N, Lévesque T, Cloutier N, Laroche A, et al. Platelets release mitochondrial antigens in systemic lupus erythematosus. Sci Transl Med. 2021;13(581). https://doi.org/10.1126/scitranslmed.aav5928. This study demonstrates how mitochondria from platelets serve as autoantigens and contribute to lupus nephritis.
Caielli S, Cardenas J, de Jesus AA, Baisch J, Walters L, Blanck JP, et al. Erythroid mitochondrial retention triggers myeloid-dependent type I interferon in human SLE. Cell. 2021. https://doi.org/10.1016/j.cell.2021.07.021. This study shows red blood cells from lupus patients still possess mitochondria, and causes type I interferon production.
Becker Y, Marcoux G, Allaeys I, Julien AS, Loignon RC, Benk-Fortin H, et al. Autoantibodies in systemic lupus erythematosus target mitochondrial RNA. Front Immunol. 2019;10:1026. https://doi.org/10.3389/fimmu.2019.01026.
Gergely P Jr, Grossman C, Niland B, Puskas F, Neupane H, Allam F, et al. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46(1):175–90. https://doi.org/10.1002/1529-0131(200201)46:1%3c175::aid-art10015%3e3.0.co;2-h.
Perl A, Gergely P Jr, Nagy G, Koncz A, Banki K. Mitochondrial hyperpolarization: a checkpoint of T-cell life, death and autoimmunity. Trends Immunol. 2004;25(7):360–7. https://doi.org/10.1016/j.it.2004.05.001.
Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem. 2003;86(5):1101–7. https://doi.org/10.1046/j.1471-4159.2003.01908.x.
Kojima M, Nakamura S, Morishita Y, Itoh H, Yoshida K, Ohno Y, et al. Reactive follicular hyperplasia in the lymph node lesions from systemic lupus erythematosus patients: a clinicopathological and immunohistological study of 21 cases. Pathol Int. 2000;50(4):304–12. https://doi.org/10.1046/j.1440-1827.2000.01052.x.
Kim J, Gupta R, Blanco LP, Yang S, Shteinfer-Kuzmine A, Wang K, et al. VDAC oligomers form mitochondrial pores to release mtDNA fragments and promote lupus-like disease. Science. 2019;366(6472):1531-6. https://doi.org/10.1126/science.aav4011. Release of mitochondrial DNA via oligomer-forming pores promotes lupus.
Emlen W, Niebur J, Kadera R. Accelerated in vitro apoptosis of lymphocytes from patients with systemic lupus erythematosus. J Immunol. 1994;152(7):3685–92.
Susin SA, Zamzami N, Castedo M, Daugas E, Wang HG, Geley S, et al. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J Exp Med. 1997;186(1):25–37. https://doi.org/10.1084/jem.186.1.25.
Banki K, Hutter E, Gonchoroff NJ, Perl A. Elevation of mitochondrial transmembrane potential and reactive oxygen intermediate levels are early events and occur independently from activation of caspases in Fas signaling. J Immunol. 1999;162(3):1466–79.
Vorobjeva N, Prikhodko A, Galkin I, Pletjushkina O, Zinovkin R, Sud’ina G, et al. Mitochondrial reactive oxygen species are involved in chemoattractant-induced oxidative burst and degranulation of human neutrophils in vitro. Eur J Cell Biol. 2017;96(3):254–65. https://doi.org/10.1016/j.ejcb.2017.03.003.
Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 2010;184(6):3284–97. https://doi.org/10.4049/jimmunol.0902199.
Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187(1):538–52. https://doi.org/10.4049/jimmunol.1100450.
Fortner KA, Blanco LP, Buskiewicz I, Huang N, Gibson PC, Cook DL, et al. Targeting mitochondrial oxidative stress with MitoQ reduces NET formation and kidney disease in lupus-prone MRL-lpr mice. Lupus Sci Med. 2020;7(1). https://doi.org/10.1136/lupus-2020-000387.
St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127(2):397–408. https://doi.org/10.1016/j.cell.2006.09.024.
Nagy G, Koncz A, Perl A. T cell activation-induced mitochondrial hyperpolarization is mediated by Ca2+- and redox-dependent production of nitric oxide. J Immunol. 2003;171(10):5188–97. https://doi.org/10.4049/jimmunol.171.10.5188.
Nagy G, Barcza M, Gonchoroff N, Phillips PE, Perl A. Nitric oxide-dependent mitochondrial biogenesis generates Ca2+ signaling profile of lupus T cells. J Immunol. 2004;173(6):3676–83. https://doi.org/10.4049/jimmunol.173.6.3676.
van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36(1):68–78. https://doi.org/10.1016/j.immuni.2011.12.007.
Ling GS, Crawford G, Buang N, Bartok I, Tian K, Thielens NM, et al. C1q restrains autoimmunity and viral infection by regulating CD8(+) T cell metabolism. Science. 2018;360(6388):558–63. https://doi.org/10.1126/science.aao4555.
Palikaras K, Lionaki E, Tavernarakis N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat Cell Biol. 2018;20(9):1013–22. https://doi.org/10.1038/s41556-018-0176-2.
Gkirtzimanaki K, Kabrani E, Nikoleri D, Polyzos A, Blanas A, Sidiropoulos P, et al. IFNα impairs autophagic degradation of mtDNA promoting autoreactivity of SLE monocytes in a STING-dependent fashion. Cell Rep. 2018;25(4):921-33.e5. https://doi.org/10.1016/j.celrep.2018.09.001.
Tiwari S, Choi HP, Matsuzawa T, Pypaert M, MacMicking JD. Targeting of the GTPase Irgm1 to the phagosomal membrane via PtdIns(3,4)P(2) and PtdIns(3,4,5)P(3) promotes immunity to mycobacteria. Nat Immunol. 2009;10(8):907–17. https://doi.org/10.1038/ni.1759.
Rai P, Janardhan KS, Meacham J, Madenspacher JH, Lin WC, Karmaus PWF, et al. IRGM1 links mitochondrial quality control to autoimmunity. Nat Immunol. 2021;22(3):312-21. https://doi.org/10.1038/s41590-020-00859-0. Autophagy/ Mitophagy regulates mitochondrial quality, and defects in mitochodrial clearance results in accumulation of mitochondrial DNA in the cytosol to trigger autoimmunity.
Oaks Z, Winans T, Caza T, Fernandez D, Liu Y, Landas SK, et al. Mitochondrial dysfunction in the liver and antiphospholipid antibody production precede disease onset and respond to rapamycin in lupus-prone mice. Arthritis Rheumatol. 2016;68(11):2728–39. https://doi.org/10.1002/art.39791.
Zhong Z, Umemura A, Sanchez-Lopez E, Liang S, Shalapour S, Wong J, et al. NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell. 2016;164(5):896–910. https://doi.org/10.1016/j.cell.2015.12.057.
Caza TN, Fernandez DR, Talaber G, Oaks Z, Haas M, Madaio MP, et al. HRES-1/Rab4-mediated depletion of Drp1 impairs mitochondrial homeostasis and represents a target for treatment in SLE. Ann Rheum Dis. 2014;73(10):1888–97. https://doi.org/10.1136/annrheumdis-2013-203794.
Alessandri C, Barbati C, Vacirca D, Piscopo P, Confaloni A, Sanchez M, et al. T lymphocytes from patients with systemic lupus erythematosus are resistant to induction of autophagy. Faseb j. 2012;26(11):4722–32. https://doi.org/10.1096/fj.12-206060.
Hubbard VM, Valdor R, Patel B, Singh R, Cuervo AM, Macian F. Macroautophagy regulates energy metabolism during effector T cell activation. J Immunol. 2010;185(12):7349–57. https://doi.org/10.4049/jimmunol.1000576.
Kabat AM, Harrison OJ, Riffelmacher T, Moghaddam AE, Pearson CF, Laing A, et al. The autophagy gene Atg16l1 differentially regulates Treg and TH2 cells to control intestinal inflammation. Elife. 2016;5: e12444. https://doi.org/10.7554/eLife.12444.
Wei J, Long L, Yang K, Guy C, Shrestha S, Chen Z, et al. Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat Immunol. 2016;17(3):277–85. https://doi.org/10.1038/ni.3365.
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275(5303):1129–32. https://doi.org/10.1126/science.275.5303.1129.
Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7(12):1166–73. https://doi.org/10.1038/sj.cdd.4400783.
Czabotar PE, Westphal D, Dewson G, Ma S, Hockings C, Fairlie WD, et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell. 2013;152(3):519–31. https://doi.org/10.1016/j.cell.2012.12.031.
Roths JB, Murphy ED, Eicher EM. A new mutation, gld, that produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J Exp Med. 1984;159(1):1–20. https://doi.org/10.1084/jem.159.1.1.
Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med. 1994;180(4):1295–306. https://doi.org/10.1084/jem.180.4.1295.
Hutcheson J, Scatizzi JC, Siddiqui AM, Haines GK 3rd, Wu T, Li QZ, et al. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity. 2008;28(2):206–17. https://doi.org/10.1016/j.immuni.2007.12.015.
Mehrian R, Quismorio FP Jr, Strassmann G, Stimmler MM, Horwitz DA, Kitridou RC, et al. Synergistic effect between IL-10 and bcl-2 genotypes in determining susceptibility to systemic lupus erythematosus. Arthritis Rheum. 1998;41(4):596–602. https://doi.org/10.1002/1529-0131(199804)41:4%3c596::aid-art6%3e3.0.co;2-2.
Andre J, Cimaz R, Ranchin B, Galambrun C, Bertrand Y, Bouvier R, et al. Overexpression of the antiapoptotic gene Bfl-1 in B cells from patients with familial systemic lupus erythematosus. Lupus. 2007;16(2):95–100. https://doi.org/10.1177/0961203306075382.
Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415(6874):922–6. https://doi.org/10.1038/415922a.
Ludwinski MW, Sun J, Hilliard B, Gong S, Xue F, Carmody RJ, et al. Critical roles of Bim in T cell activation and T cell-mediated autoimmune inflammation in mice. J Clin Invest. 2009;119(6):1706–13. https://doi.org/10.1172/jci37619.
Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J Exp Med. 2003;198(7):1119–26. https://doi.org/10.1084/jem.20030411.
Labi V, Erlacher M, Kiessling S, Manzl C, Frenzel A, O’Reilly L, et al. Loss of the BH3-only protein Bmf impairs B cell homeostasis and accelerates gamma irradiation-induced thymic lymphoma development. J Exp Med. 2008;205(3):641–55. https://doi.org/10.1084/jem.20071658.
Thompson J, Winoto A. During negative selection, Nur77 family proteins translocate to mitochondria where they associate with Bcl-2 and expose its proapoptotic BH3 domain. J Exp Med. 2008;205(5):1029–36. https://doi.org/10.1084/jem.20080101.
Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, et al. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116(4):527–40. https://doi.org/10.1016/s0092-8674(04)00162-x.
Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, et al. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 2002;16(6):759–67. https://doi.org/10.1016/s1074-7613(02)00322-9.
Bauer A, Villunger A, Labi V, Fischer SF, Strasser A, Wagner H, et al. The NF-kappaB regulator Bcl-3 and the BH3-only proteins Bim and Puma control the death of activated T cells. Proc Natl Acad Sci U S A. 2006;103(29):10979–84. https://doi.org/10.1073/pnas.0603625103.
Fischer SF, Bouillet P, O’Donnell K, Light A, Tarlinton DM, Strasser A. Proapoptotic BH3-only protein Bim is essential for developmentally programmed death of germinal center-derived memory B cells and antibody-forming cells. Blood. 2007;110(12):3978–84. https://doi.org/10.1182/blood-2007-05-091306.
Mietzner B, Tsuiji M, Scheid J, Velinzon K, Tiller T, Abraham K, et al. Autoreactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci U S A. 2008;105(28):9727–32. https://doi.org/10.1073/pnas.0803644105.
Acknowledgements
The authors wish to thank Dr. Qianjin Lu for reviewing their manuscript.
Author information
Authors and Affiliations
Contributions
Ping-Min Chen wrote the manuscript. George Tsokos supervised and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not involve human subjects or animal protocol necessary for ethics approval.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Systemic Lupus Erythematosus
Rights and permissions
About this article
Cite this article
Chen, PM., Tsokos, G.C. Mitochondria in the Pathogenesis of Systemic Lupus Erythematosus. Curr Rheumatol Rep 24, 88–95 (2022). https://doi.org/10.1007/s11926-022-01063-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-022-01063-9