Abstract
Purpose of Review
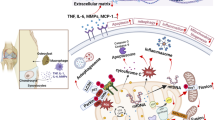
This review will cover foundational studies and recent findings that established key concepts for understanding the importance of redox biology to chondrocyte mitochondrial function and osteoarthritis pathophysiology after injury.
Recent Findings
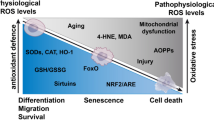
Articular chondrocyte mitochondria can be protected with a wide variety of antioxidants that will be discussed within a framework suggested by classic studies. These agents not only underscore the importance of thiol metabolism and associated redox function for chondrocyte mitochondria but also suggest complex interactions with signal transduction pathways and other molecular features of osteoarthritis that require more thorough investigation. Emerging evidence also indicates that reductive stress could occur alongside oxidative stress.
Summary
Recent studies have shed new light on historic paradoxes in chondrocyte redox and mitochondrial physiology, leading to the development of promising disease-modifying therapies for posttraumatic osteoarthritis.

Similar content being viewed by others
References
Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504.
Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97(1):33–44.
Onyekwelu I, Goldring MB, Hidaka C. Chondrogenesis, joint formation, and articular cartilage regeneration. J Cell Biochem. 2009;107(3):383–92.
Lin Z, Willers C, Xu J, Zheng MH. The chondrocyte: biology and clinical application. Tissue Eng. 2006;12(7):1971–84.
Hunziker EB, Schenk RK, Cruz-Orive LM. Quantitation of chondrocyte performance in growth-plate cartilage during longitudinal bone growth. J Bone Joint Surg Am. 1987;69(2):162–73.
Hunziker EB, Kapfinger E, Geiss J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthr Cartil. 2007;15(4):403–13.
Archer CW, Francis-West P. The chondrocyte. Int J Biochem Cell Biol. 2003;35(4):401–4.
Eyre D. Collagen of articular cartilage. Arthritis Res. 2002;4(1):30–5.
Eggli PS, Herrmann W, Hunziker EB, Schenk RK. Matrix compartments in the growth plate of the proximal tibia of rats. Anat Rec. 1985;211(3):246–57.
Vincent TL. Targeting mechanotransduction pathways in osteoarthritis: a focus on the pericellular matrix. Curr Opin Pharmacol. 2013;13(3):449–54.
Buckwalter JA, Mankin HJ. Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect. 1998; 47:477–86
Lohmander S. Proteoglycans of joint cartilage. Structure, function, turnover and role as markers of joint disease. Baillieres Clin Rheumatol. 1988;2(1):37–62.
Benninghoff A. Form und Bau der Gelenkknorpel in ihren Beziehungen Zur Funktion. Zweiter Teil: der Aufbau des Gelenkknorpels in sienen Bezienhungen zur Funktion., (2) (1925) 783-862.
Guilak F, Alexopoulos LG, Upton ML, Youn I, Choi JB, Cao L, et al. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Ann N Y Acad Sci. 2006;1068:498–512.
Youn I, Choi JB, Cao L, Setton LA, Guilak F. Zonal variations in the three-dimensional morphology of the chondron measured in situ using confocal microscopy. Osteoarthr Cartil. 2006;14(9):889–97.
Knudson CB, Knudson W. Cartilage proteoglycans. Semin Cell Dev Biol. 2001;12(2):69–78.
Charlier E, Deroyer C, Ciregia F, Malaise O, Neuville S, Plener Z, et al. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem Pharmacol. 2019;165:49–65.
Goldring MB. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Pract Res Clin Rheumatol. 2006;20(5):1003–25.
Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–69.
Buckwalter JA. Articular cartilage. Instr Course Lect. 1983;32:349–70.
Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461–8.
Freemont AJ. Microscopic analysis of synovial fluid—the perfect diagnostic test? Ann Rheum Dis. 1996;55(10):695–7.
Maroudas A, Bullough P, Swanson SA, Freeman MA. The permeability of articular cartilage. J Bone Joint Surg (Br). 1968;50(1):166–77.
Maroudas A. Distribution and diffusion of solutes in articular cartilage. Biophys J. 1970;10(5):365–79.
Mobasheri A. Glucose: an energy currency and structural precursor in articular cartilage and bone with emerging roles as an extracellular signaling molecule and metabolic regulator. Front Endocrinol (Lausanne). 2012;3:153.
Clark AG, Rohrbaugh AL, Otterness I, Kraus VB. The effects of ascorbic acid on cartilage metabolism in guinea pig articular cartilage explants. Matrix Biol. 2002;21(2):175–84.
Mobasheri A, Vannucci SJ, Bondy CA, Carter SD, Innes JF, Arteaga MF, et al. Glucose transport and metabolism in chondrocytes: a key to understanding chondrogenesis, skeletal development and cartilage degradation in osteoarthritis. Histol Histopathol. 2002;17(4):1239–67.
Zhang W, Likhodii S, Aref-Eshghi E, Zhang Y, Harper PE, Randell E, et al. Relationship between blood plasma and synovial fluid metabolite concentrations in patients with osteoarthritis. J Rheumatol. 2015;42(5):859–65.
Mobasheri A, Neama G, Bell S, Richardson S, Carter SD. Human articular chondrocytes express three facilitative glucose transporter isoforms: GLUT1, GLUT3 and GLUT9. Cell Biol Int. 2002;26(3):297–300.
Heywood HK, Knight MM, Lee DA. Both superficial and deep zone articular chondrocyte subpopulations exhibit the Crabtree effect but have different basal oxygen consumption rates. J Cell Physiol. 2010;223(3):630–9.
Blanco FJ, López-Armada MJ, Maneiro E. Mitochondrial dysfunction in osteoarthritis. Mitochondrion. 2004;4(5-6):715–28.
Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instr Course Lect. 2005;54:465–80.
Lee RB, Urban JP. Evidence for a negative Pasteur effect in articular cartilage. Biochem J. 1997;321(Pt 1):95–102.
Joost HG, Thorens B. The extended GLUT-family of sugar/polyol transport facilitators: nomenclature, sequence characteristics, and potential function of its novel members (review). Mol Membr Biol. 2001;18(4):247–56.
Rosa SC, Gonçalves J, Judas F, Mobasheri A, Lopes C, Mendes AF. Impaired glucose transporter-1 degradation and increased glucose transport and oxidative stress in response to high glucose in chondrocytes from osteoarthritic versus normal human cartilage. Arthritis Res Ther. 2009;11(3):R80.
Wang J, Zhou J, Bondy CA. Igf1 promotes longitudinal bone growth by insulin-like actions augmenting chondrocyte hypertrophy. FASEB J. 1999;13(14):1985–90.
Peansukmanee S, Vaughan-Thomas A, Carter SD, Clegg PD, Taylor S, Redmond C, et al. Effects of hypoxia on glucose transport in primary equine chondrocytes in vitro and evidence of reduced GLUT1 gene expression in pathologic cartilage in vivo. J Orthop Res. 2009;27(4):529–35.
Marcus RE. The effect of low oxygen concentration on growth, glycolysis, and sulfate incorporation by articular chondrocytes in monolayer culture. Arthritis Rheum. 1973;16(5):646–56.
Lee RB, Urban JP. Functional replacement of oxygen by other oxidants in articular cartilage. Arthritis Rheum. 2002;46(12):3190–200.
Lee RB, Wilkins RJ, Razaq S, Urban JP. The effect of mechanical stress on cartilage energy metabolism. Biorheology. 2002;39(1-2):133–43.
Johnson K, Jung A, Murphy A, Andreyev A, Dykens J, Terkeltaub R. Mitochondrial oxidative phosphorylation is a downstream regulator of nitric oxide effects on chondrocyte matrix synthesis and mineralization. Arthritis Rheum. 2000;43(7):1560–70.
Guri CD, Bernstein DS. Rat epiphyseal cartilage. V. Glucose-C14 metabolism as related to growth and to various anatomical areas, in vitro. Proc Soc Exp Biol Med. 1967;124(2):386–91.
Stockwell RA. Metabolism of cartilage. In: Hall BK, editor. Cartilage, structure, function, and biochemistry. New York: New York Academic Press; 1983. p. 253–80.
Grimshaw MJ, Mason RM. Modulation of bovine articular chondrocyte gene expression in vitro by oxygen tension. Osteoarthr Cartil. 2001;9(4):357–64.
Wolff KJ, Ramakrishnan PS, Brouillette MJ, Journot BJ, McKinley TO, Buckwalter JA, et al. Mechanical stress and ATP synthesis are coupled by mitochondrial oxidants in articular cartilage. J Orthop Res. 2013;31(2):191–6.
Martin JA, Martini A, Molinari A, Morgan W, Ramalingam W, Buckwalter JA, et al. Mitochondrial electron transport and glycolysis are coupled in articular cartilage. Osteoarthr Cartil. 2012;20(4):323–9.
Ohashi T, Hagiwara M, Bader DL, Knight MM. Intracellular mechanics and mechanotransduction associated with chondrocyte deformation during pipette aspiration. Biorheology. 2006;43(3,4):201–14.
Lv M, Zhou Y, Chen X, Han L, Wang L, Lu XL. Calcium signaling of in situ chondrocytes in articular cartilage under compressive loading: roles of calcium sources and cell membrane ion channels. J Orthop Res. 2018;36(2):730–8.
Lee W, Guilak F, Liedtke W. Role of piezo channels in joint health and injury. Curr Top Membr. 2017;79:263–73.
Grandolfo M, Calabrese A, D’Andrea P. Mechanism of mechanically induced intercellular calcium waves in rabbit articular chondrocytes and in HIG-82 synovial cells. J Bone Miner Res. 1998;13(3):443–53.
Raizman I, De Croos JN, Pilliar R, Kandel RA. Calcium regulates cyclic compression-induced early changes in chondrocytes during in vitro cartilage tissue formation. Cell Calcium. 2010;48(4):232–42.
Zelenski NA, Leddy HA, Sanchez-Adams J, Zhang J, Bonaldo P, Liedtke W, et al. Type VI collagen regulates pericellular matrix properties, chondrocyte swelling, and mechanotransduction in mouse articular cartilage. Arthritis Rheum. 2015;67(5):1286–94.
Parvizi J, Parpura V, Greenleaf JF, Bolander ME. Calcium signaling is required for ultrasound-stimulated aggrecan synthesis by rat chondrocytes. J Orthop Res. 2002;20(1):51–7.
Clark AL, Votta BJ, Kumar S, Liedtke W, Guilak F. Chondroprotective role of the osmotically sensitive ion channel transient receptor potential vanilloid 4: age- and sex-dependent progression of osteoarthritis in Trpv4-deficient mice. Arthritis Rheum. 2010;62(10):2973–83.
Sánchez JC, López-Zapata DF, Wilkins RJ. TRPV4 channels activity in bovine articular chondrocytes: regulation by obesity-associated mediators. Cell Calcium. 2014;56(6):493–503.
Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009;60(10):3028–37.
Buckwalter JA, Mower D, Ungar R, Schaeffer J, Ginsberg B. Morphometric analysis of chondrocyte hypertrophy. J Bone Joint Surg Am. 1986;68(2):243–55.
Mignotte F, Champagne AM, Froger-Gaillard B, Benel L, Gueride M, Adolphe M, et al. Mitochondrial biogenesis in rabbit articular chondrocytes transferred to culture. Biol Cell. 1991;71(1-2):67–72.
Brighton CT, Kitajima T, Hunt RM. Zonal analysis of cytoplasmic components of articular cartilage chondrocytes. Arthritis Rheum. 1984;27(11):1290–9.
Zhou S, Cui Z, Urban JP. Factors influencing the oxygen concentration gradient from the synovial surface of articular cartilage to the cartilage-bone interface: a modeling study. Arthritis Rheum. 2004;50(12):3915–24.
Fermor B, Christensen SE, Youn I, Cernanec JM, Davies CM, Weinberg JB. Oxygen, nitric oxide and articular cartilage. Eur Cell Mater. 2007;13:56–65 discussion 65.
Krebs HA. The Pasteur effect and the relations between respiration and fermentation. Essays Biochem. 1972;8:1–34.
Sampson HW, Cannon MS. Zonal analysis of metabolic profiles of articular-epiphyseal cartilage chondrocytes: a histochemical study. Histochem J. 1986;18(5):233–8.
Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–8.
Sarsour EH, Kalen AL, Goswami PC. Manganese superoxide dismutase regulates a redox cycle within the cell cycle. Antioxid Redox Signal. 2014;20(10):1618–27.
Arra M, Swarnkar G, Ke K, Otero JE, Ying J, Duan X, et al. LDHA-mediated ROS generation in chondrocytes is a potential therapeutic target for osteoarthritis. Nat Commun. 2020;11(1):3427.
Chandel NS. Evolution of mitochondria as signaling organelles. Cell Metab. 2015;22(2):204–6.
Tielens AG, Rotte C, van Hellemond JJ, Martin W. Mitochondria as we don’t know them. Trends Biochem Sci. 2002;27(11):564–72.
Delco ML, Bonnevie ED, Bonassar LJ, Fortier LA. Mitochondrial dysfunction is an acute response of articular chondrocytes to mechanical injury. J Orthop Res. 2018;36(2):739–50.
Coleman MC, Ramakrishnan PS, Brouillette MJ, Martin JA. Injurious loading of articular cartilage compromises chondrocyte respiratory function. Arthritis Rheum. 2016;68(3):662–71.
Halliwell B. Oxidative stress in cell culture: an under-appreciated problem? FEBS Lett. 2003;540(1-3):3–6.
Packer L, Fuehr K. Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature. 1977;267(5610):423–5.
Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300(2):535–43.
Goodwin W, McCabe D, Sauter E, Reese E, Walter M, Buckwalter JA, et al. Rotenone prevents impact-induced chondrocyte death. J Orthop Res. 2010;28(8):1057–63.
Brouillette MJ, Ramakrishnan PS, Wagner VM, Sauter EE, Journot BJ, McKinley TO, et al. Strain-dependent oxidant release in articular cartilage originates from mitochondria. Biomech Model Mechanobiol. 2014;13(3):565–72.
Ferreira R, Llesuy S, Milei J, Scordo D, Hourquebie H, Molteni L, et al. Assessment of myocardial oxidative stress in patients after myocardial revascularization. Am Heart J. 1988;115(2):307–12.
Buettner GR, Ng CF, Wang M, Rodgers VG, Schafer FQ. A new paradigm: manganese superoxide dismutase influences the production of H2O2 in cells and thereby their biological state. Free Radic Biol Med. 2006;41(8):1338–50.
Coleman MC, Brouillette MJ, Andresen NS, Oberley-Deegan RE, Martin JM. Differential effects of superoxide dismutase mimetics after mechanical overload of articular cartilage. Antioxidants (Basel). 2017;6(4):98.
Coleman MC, Goetz JE, Brouillette MJ, Seol D, Willey MC, Petersen EB, et al. Targeting mitochondrial responses to intra-articular fracture to prevent posttraumatic osteoarthritis. Sci Transl Med. 2018;10(427):eaan5372.
Caramés B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62(3):791–801.
Vaillancourt F, Fahmi H, Shi Q, Lavigne P, Ranger P, Fernandes JC, et al. 4-Hydroxynonenal induces apoptosis in human osteoarthritic chondrocytes: the protective role of glutathione-S-transferase. Arthritis Res Ther. 2008;10(5):R107.
Carlo MD, Loeser RF. Increased oxidative stress with aging reduces chondrocyte survival: correlation with intracellular glutathione levels. Arthritis Rheum. 2003;48(12):3419–30.
Goetz JE, Coleman MC, Fredericks DC, Petersen E, Martin JA, McKinley TO, et al. Time-dependent loss of mitochondrial function precedes progressive histologic cartilage degeneration in a rabbit meniscal destabilization model. J Orthop Res. 2017;35(3):590–9.
Moussavi-Harami SF, Pedersen DR, Martin JA, Hillis SL, Brown TD. Automated objective scoring of histologically apparent cartilage degeneration using a custom image analysis program. J Orthop Res. 2009;27(4):522–8.
Pedersen DR, Goetz JE, Kurriger GL, Martin JA. Comparative digital cartilage histology for human and common osteoarthritis models. Orthop Res Rev. 2013;2013(5):13–20.
Goetz JE, Fredericks D, Petersen E, Rudert MJ, Baer T, Swanson E, et al. A clinically realistic large animal model of intra-articular fracture that progresses to post-traumatic osteoarthritis. Osteoarthr Cartil. 2015;23(10):1797–805.
Huser CA, Davies ME. Calcium signaling leads to mitochondrial depolarization in impact-induced chondrocyte death in equine articular cartilage explants. Arthritis Rheum. 2007;56(7):2322–34.
Abusara Z, Von Kossel M, Herzog W. In vivo dynamic deformation of articular cartilage in intact joints loaded by controlled muscular contractions. PLoS One. 2016;11(1):e0147547.
Han SK, Ronkainen AP, Saarakkala S, Rieppo L, Herzog W, Korhonen RK. Alterations in structural macromolecules and chondrocyte deformations in lapine retropatellar cartilage 9 weeks after anterior cruciate ligament transection. J Orthop Res. 2018;36(1):342–50.
Collins JA, Wood ST, Bolduc JA, Nurmalasari NPD, Chubinskaya S, Poole LB, et al. Differential peroxiredoxin hyperoxidation regulates MAP kinase signaling in human articular chondrocytes. Free Radic Biol Med. 2019;134:139–52.
Delco ML, Bonnevie ED, Szeto HS, Bonassar LJ, Fortier LA. Mitoprotective therapy preserves chondrocyte viability and prevents cartilage degeneration in an ex vivo model of posttraumatic osteoarthritis. J Orthop Res. 2018;36:2147–56.
López de Figueroa P, Lotz MK, Blanco FJ, Caramés B. Autophagy activation and protection from mitochondrial dysfunction in human chondrocytes. Arthritis Rheum. 2015;67(4):966–76.
Ansari MY, Ball HC, Wase SJ, Novak K, Haqqi TM. Lysosomal dysfunction in osteoarthritis and aged cartilage triggers apoptosis in chondrocytes through BAX mediated release of cytochrome c. Osteoarthr Cartil. 2020;28:S67.
Ansari MY, Ahmad N, Voleti S, Wase SJ, Novak K, Haqqi TM. Mitochondrial dysfunction triggers a catabolic response in chondrocytes via ROS-mediated activation of the JNK/AP1 pathway. J Cell Sci. 2020;133(22):jcs247353.
Vaamonde-García C, Loureiro J, Valcárcel-Ares MN, Riveiro-Naveira RR, Ramil-Gómez O, Hermida-Carballo L, et al. The mitochondrial inhibitor oligomycin induces an inflammatory response in the rat knee joint. BMC Musculoskelet Disord. 2017;18(1):254.
Vaamonde-García C, Riveiro-Naveira RR, Valcárcel-Ares MN, Hermida-Carballo L, Blanco FJ, López-Armada MJ. Mitochondrial dysfunction increases inflammatory responsiveness to cytokines in normal human chondrocytes. Arthritis Rheum. 2012;64(9):2927–36.
Cillero-Pastor B, Caramés B, Lires-Deán M, Vaamonde-García C, Blanco FJ, López-Armada MJ. Mitochondrial dysfunction activates cyclooxygenase 2 expression in cultured normal human chondrocytes. Arthritis Rheum. 2008;58(8):2409–19.
Blanco FJ, June RK. Cartilage metabolism, mitochondria, and osteoarthritis. J Am Acad Orthop Surg. 2020;28(6):e242–4.
Blanco FJ, Rego I, Ruiz-Romero C. The role of mitochondria in osteoarthritis. Nat Rev Rheumatol. 2011;7(3):161–9.
Wu L, Liu H, Li L, Cheng Q, Li H, Huang H. Mitochondrial pathology in osteoarthritic chondrocytes. Curr Drug Targets. 2014;15(7):710–9.
Canter PH, Wider B, Ernst E. The antioxidant vitamins A, C, E and selenium in the treatment of arthritis: a systematic review of randomized clinical trials. Rheumatology (Oxford). 2007;46(8):1223–33.
Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36(10):1199–207.
Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266(18):11632–9.
Dhakshinamoorthy S, Jain AK, Bloom DA, Jaiswal AK. Bach1 competes with Nrf2 leading to negative regulation of the antioxidant response element (ARE)-mediated NAD(P)H:quinone oxidoreductase 1 gene expression and induction in response to antioxidants. J Biol Chem. 2005;280(17):16891–900.
Davidson RK, Jupp O, de Ferrars R, Kay CD, Culley KL, Norton R, et al. Sulforaphane represses matrix-degrading proteases and protects cartilage from destruction in vitro and in vivo. Arthritis Rheum. 2013;65(12):3130–40.
Khan NM, Haseeb A, Ansari MY, Devarapalli P, Haynie S, Haqqi TM. Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human osteoarthritis chondrocytes. Free Radic Biol Med. 2017;106:288–301.
Mével E, Merceron C, Vinatier C, Krisa S, Richard T, Masson M, et al. Olive and grape seed extract prevents post-traumatic osteoarthritis damages and exhibits in vitro anti IL-1β activities before and after oral consumption. Sci Rep. 2016;6:33527.
Wang A, Leong DJ, He Z, Xu L, Liu L, Kim SJ, et al. Procyanidins mitigate osteoarthritis pathogenesis by, at least in part, suppressing vascular endothelial growth factor signaling. Int J Mol Sci. 2016 Dec 9;17(12):2065.
Basu A, Schell J, Scofield RH. Dietary fruits and arthritis. Food Funct. 2018;9(1):70–7.
Leong DJ, Choudhury M, Hanstein R, Hirsh DM, Kim SJ, Majeska RJ, et al. Green tea polyphenol treatment is chondroprotective, anti-inflammatory and palliative in a mouse post-traumatic osteoarthritis model. Arthritis Res Ther. 2014;16(6):508.
Vaamonde-García C, Burguera EF, Vela-Anero Á, Hermida-Gómez T, Filgueira-Fernández P, Fernández-Rodríguez JA, et al. Intraarticular administration effect of hydrogen sulfide on an in vivo rat model of osteoarthritis. Int J Mol Sci. 2020 Oct 8;21(19):7421.
O'Grady KP, Kavanaugh TE, Cho H, Ye H, Gupta MK, Madonna MC, et al. Drug-free ROS sponge polymeric microspheres reduce tissue damage from ischemic and mechanical injury. ACS Biomater Sci Eng. 2018;4(4):1251–64.
Lepetsos P, Papavassiliou AG. ROS/oxidative stress signaling in osteoarthritis. Biochim Biophys Acta. 2016;1862(4):576–91.
Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47(9):1304–9.
Shao Z, Pan Z, Lin J, Zhao Q, Wang Y, Ni L, et al. S-allyl cysteine reduces osteoarthritis pathology in the tert-butyl hydroperoxide-treated chondrocytes and the destabilization of the medial meniscus model mice via the Nrf2 signaling pathway. Aging (Albany NY). 2020;12(19):19254–72.
Yan Z, Qi W, Zhan J, Lin Z, Lin J, Xue X, et al. Activating Nrf2 signalling alleviates osteoarthritis development by inhibiting inflammasome activation. J Cell Mol Med. 2020;24:13046–57.
Yang J, Song X, Feng Y, Liu N, Fu Z, Wu J, et al. Natural ingredients-derived antioxidants attenuate H. Free Radic Biol Med. 2020;152:854–64.
Chen Z, Zhong H, Wei J, Lin S, Zong Z, Gong F, et al. Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Res Ther. 2019;21(1):300.
Khan NM, Ahmad I, Haqqi TM. Nrf2/ARE pathway attenuates oxidative and apoptotic response in human osteoarthritis chondrocytes by activating ERK1/2/ELK1-P70S6K-P90RSK signaling axis. Free Radic Biol Med. 2018;116:159–71.
Wang Y, Zhao X, Lotz M, Terkeltaub R, Liu-Bryan R. Mitochondrial biogenesis is impaired in osteoarthritis chondrocytes but reversible via peroxisome proliferator-activated receptor γ coactivator 1α. Arthritis Rheum. 2015;67(8):2141–53.
Takada T, Miyaki S, Ishitobi H, Hirai Y, Nakasa T, Igarashi K, et al. Bach1 deficiency reduces severity of osteoarthritis through upregulation of heme oxygenase-1. Arthritis Res Ther. 2015;17:285.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gomez-Contreras, P.C., Kluz, P.N., Hines, M.R. et al. Intersections Between Mitochondrial Metabolism and Redox Biology Mediate Posttraumatic Osteoarthritis. Curr Rheumatol Rep 23, 32 (2021). https://doi.org/10.1007/s11926-021-00994-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s11926-021-00994-z