Abstract
Purpose of Review
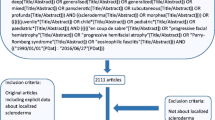
Treatment of scleroderma in children is challenging since little is known about its pathogenesis. Herein, we review the most recent evidence regarding the treatment of juvenile scleroderma.
Recent Findings
According to the recent recommendations for Pediatric Rheumatology in Europe (SHARE), systemic treatment in localized scleroderma is needed when there is a risk for disability, such as in generalized or pansclerotic morphea and progressive linear scleroderma. In juvenile systemic sclerosis, the introduction of the severity score, J4S, has standardized the assessment of the patients in the daily practice and allowed a more tailored therapeutic approach. Since, to date, no clinical trial is available in JSSc, due to its rarity, the treatment is based on adults’ experience.
Summary
The recent recommendations for juvenile scleroderma represent an important instrument to standardize the treatment approach, confirm the role of methotrexate, and open new windows for effective experimental treatments, such as mycophenolate mofetil and biological agents, for severe or refractory cases.

Similar content being viewed by others
Notes
Martini G, Saggioro L, Culpo R, Vittadello F, Meneghel A, Zulian F. Mycophenolate mofetil for methotrexate-resistant Juvenile Localised Scleroderma. Rheumatology (Oxford) 2020 (submitted).
Foeldvari I, Culpo R, Sperotto F, et al. Consensus-based recommendations for the management of Juvenile Systemic Sclerosis. Rheumatology (Oxford) 2020 (submitted).
Khanna D, Lin CJF, Kuwana M, et al. Efficacy and safety of tocilizumab for the treatment of systemic sclerosis: results from a phase 3 randomized controlled trial [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 10).
Bhattacharyya S, Wang W, Wie J, et al. Pharmacological inhibition of JAK/STAT signaling by tofacitinib prevents experimental organ fibrosis: novel therapy for systemic sclerosis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 10).
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Denton CP, Derrett-Smith EC. Juvenile-onset systemic sclerosis: children are not small adults. Rheumatology (Oxford). 2009;48(2):96–7. https://doi.org/10.1093/rheumatology/ken418.
Zulian F, Vallongo C, de Oliveira SKF, Punaro MG, Ros J, Mazur-Zielinska H, et al. Congenital localized Scleroderma. J Pediatr. 2006;149(2):248–51. https://doi.org/10.1016/j.jpeds.2006.04.052.
Zulian F, Athreya BH, Laxer RM, Nelson AM, de Oliveira SKF, Punaro MG, et al. Juvenile Localized Scleroderma: clinical and epidemiological features in 750 children. An international study. Rheumatology (Oxford). 2006;45(5):614–20. https://doi.org/10.1093/rheumatology/kei251.
Zulian F, Vallongo C, Woo P, Russo R, Ruperto N, Harper J, et al. Localized Scleroderma in childhood is not just a skin disease. Arthritis Rheum. 2005;52(9):2873–81. https://doi.org/10.1002/art.21264.
•• Torok KS, Li SC, Jacobe HM, Taber SF, Stevens AM. Zulian F Immunopathogenesis of Pediatric Localized Scleroderma. Front. Immunol. 2019;10:908. https://doi.org/10.3389/fimmu.2019.00908Review on recent findings on the immunopathogenesis of juvenile localized scleroderma underlying the elevated circulating chemokines and cytokines levels associated with T-helper cell, IFNγ, and other inflammatory pathways. The unique features of the immune system in childhood may potentially contribute to some of the differences between children and adults.
Zulian F. Scleroderma in children. Best Pract Res Clin Rheumatol. 2017;31(4):576–95. https://doi.org/10.1016/j.berh.2018.02.004.
Laxer RM, Zulian F. Localized scleroderma. Curr Opin Rheumatol. 2006 Nov;18(6):606–13.
Constantin T, Foeldvari I, Pain CE, Pálinkás A, Höger P, Moll M, et al. Development of minimum standards of care for juvenile localized scleroderma. Eur J Pediatr. 2018;177(7):961–77. https://doi.org/10.1007/s00431-018-3144-8.
•• Zulian F, Culpo R, Sperotto F, Anton J, Avcin T, Baildam EM, et al. Consensus-based recommendations for the management of juvenile localised scleroderma. Ann Rheum Dis. 2019;78(8):1019–24.
Zulian F, Martini G, Vallongo C, Vittadello F, Falcini F, Patrizi A, et al. Methotrexate treatment in juvenile localized scleroderma: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2011;63(7):1998–2006. https://doi.org/10.1002/art.30264.
Uziel Y, Feldman BM, Krafchik BR, Yeung RS, Laxer RM. Methotrexate and corticosteroid therapy for pediatric localized scleroderma. J Pediatr. 2000;136(1):91–5.
Weibel L, Sampaio MC, Visentin MT, Howell KJ, Woo P, Harper JI. Evaluation of methotrexate and corticosteroids for the treatment of localized scleroderma (morphoea) in children. Br J Dermatol. 2006;155(5):1013–20.
Torok KS, Arkachaisri T. Methotrexate and corticosteroids in the treatment of localized scleroderma: a standardized prospective longitudinal single-center study. J Rheumatol. 2012;39(2):286–94. https://doi.org/10.3899/jrheum.110210.
Li SC, Torok KS, Pope E, Dedeoglu F, Hong S, Jacobe HT, et al. Development of consensus treatment plans for juvenile localized scleroderma: a roadmap toward comparative effectiveness studies in juvenile localized scleroderma. Arthritis Care Res (Hoboken). 2012;64(8):1175–85. https://doi.org/10.1002/acr.21687.
• Lythgoe H, Almeida B, Bennett J, et al. Multi-Centre national audit of juvenile localised scleroderma: describing current UK practice in disease assessment and management. Pediatr Rheumatol online J. 2018;16(1):80. https://doi.org/10.1186/s12969-018-0295-0Study describing the current approach, in terms of assessment and treatment, to juvenile localized scleroderma in the UK. It underlines the need to better define outcome measures and treatment regimens for JLS.
Anderson LE, Treat JR, Licht DJ, Kreiger PA, Knight AM. Remission of seizures with immunosuppressive therapy in Parry-Romberg syndrome and en coup de sabre linear scleroderma: case report and brief review of the literature. Pediatr Dermatol. 2018;35(6):e363–5. https://doi.org/10.1111/pde.13647.
Zulian F, Vallongo C, Patrizi A, Belloni-Fortina A, Cutrone M, Alessio M, et al. A long-term follow-up study of methotrexate in juvenile localized scleroderma (morphea). J Am Acad Dermatol. 2012;67(6):1151–6. https://doi.org/10.1016/j.jaad.2012.03.036.
• Hardy J, Boralevi F, Mallet S, et al. Clinical Profile of Methotrexate-resistant Juvenile Localised Scleroderma. Acta Derm Venereol. 2019;99(6):539–43. https://doi.org/10.2340/00015555-3155Retrospective multicenter study of the French Society of Paediatric Dermatology confirming the efficacy of methotrexate of juvenile localized scleroderma with less than 10% resistance to treatment.
Mirsky L, Chakkittakandiyil A, Laxer RM, O’Brien C, Pope E. Relapse after systemic treatment in paediatric morphoea. Br J Dermatol. 2012;166(2):443–5. https://doi.org/10.1111/j.1365-2133.2011.10535.x.
Martini G, Ramanan AV, Falcini F, Girschick H, Goldsmith DP, Zulian F. Successful treatment of severe or methotrexate-resistant juvenile localized scleroderma with mycophenolate mofetil. Rheumatology (Oxford). 2009;48(11):1410–3. https://doi.org/10.1093/rheumatology/kep244.
Mertens JS, Marsman D, van de Kerkhof PC, et al. Use of mycophenolate mofetil in patients with severe localized scleroderma resistant or intolerant to methotrexate. Acta Derm Venereol. 2016;96(4):510–3. https://doi.org/10.2340/00015555-2297.
•• Martini G, Campus S, Raffeiner B, Boscarol G, Meneghel A, Zulian F. Tocilizumab in Two Children With Pansclerotic Morphoea: A Hopeful Therapy for Refractory Cases? Clin Exp Rheumatol. 2017;35(Suppl 106(4)):211–3 Description of the first two patients with pansclerotic morphoea, an extremely severe subtype of localized scleroderma, in which tocilizumab, a fully humanized anti IL-6R antibody, allowed to control inflammation and stopped the extension of the disease.
• Lythgoe H, Baildam E, Beresford MW, Cleary G, McCann LJ, Pain CE. Tocilizumab as a potential therapeutic option for children with severe, refractory juvenile localized scleroderma. Rheumatology (Oxford). 2018;57(2):398–401. https://doi.org/10.1093/rheumatology/kex382Study confirming the efficacy of tocilizumab, a fully humanized anti IL-6R antibody, as a potential therapeutic option for children with severe, refractory juvenile localized scleroderma.
Ponsoye M, Frantz C, Ruzehaji N, Nicco C, Elhai M, Ruiz B, et al. Treatment with abatacept prevents experimental dermal fibrosis and induces regression of established inflammation-driven fibrosis. Ann Rheum Dis. 2016;75(12):2142–9. https://doi.org/10.1136/annrheumdis-2015-208213.
Stausbøl-Grøn B, Olesen AB, Deleuran B, Deleuran MS. Abatacept is a promising treatment for patients with disseminated morphea profunda: presentation of two cases. Acta Derm Venereol. 2011;91(6):686–8. https://doi.org/10.2340/00015555-1136.
• Fage SW, Arvesen KB, Olesen AB. Abatacept Improves Skin-score and Reduces Lesions in Patients with Localized Scleroderma: A Case Series. Acta Derm Venereol. 2018;98(4):465–6. https://doi.org/10.2340/00015555-2878Study describing a case series of 13 adult patients with localized scleroderma successfully treated with abatacept, a selective T cell co-stimulation modulator.
Hassani J, Feldman SR. Phototherapy in scleroderma. Dermatol Ther (Heidelb). 2016;6(4):519–53. https://doi.org/10.1007/s13555-016-0136-3.
Marrani E, Foeldvari I, Jordi AP, Cimaz R, Simonini G. Comparing ultraviolet light a photo(chemo)therapy with methotrexate protocol in childhood localized scleroderma: evidence from systematic review and meta-analysis approach. Semin Arthritis Rheum. 2018;48(3):495–503. https://doi.org/10.1016/j.semarthrit.2018.03.003.
Pope E, Doria AS, Theriault M, Mohanta A, Laxer RM. Topical imiquimod 5% cream for pediatric plaque morphea: a prospective, multiple-baseline, open-label pilot study. Dermatology. 2011;223(4):363–9. https://doi.org/10.1159/000335560.
Dytoc M, Wat H, Cheung-Lee M, Sawyer D, Ackerman T, Fiorillo L. Evaluation of the efficacy and safety of topical imiquimod 5% for plaque-type morphea: a multicenter, prospective, vehicle-controlled trial. J Cutan Med Surg. 2015;19(2):132–9. https://doi.org/10.2310/7750.2014.14072.
Herrick AL, Ennis H, Bhushan M, Silma AJ, Baidam EM. Incidence of childhood linear scleroderma and systemic sclerosis in the UK and Ireland. Arthritis Care Res (Hoboken). 2010;62(2):213–8. https://doi.org/10.1002/acr.20070.
Mayes MD, Lacey JV Jr, Beebe-Dimmer J, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48(8):2246–55.
Martini G, Vittadello F, Kasapçopur O, et al. Factors affecting survival in juvenile systemic sclerosis. Rheumatology (Oxford). 2009;48(2):119–22. https://doi.org/10.1093/rheumatology/ken388.
Martini G, Foeldvari I, Russo R, Cuttica R, Eberhard A, Ravelli A, et al. Systemic sclerosis in childhood: clinical and immunological features of 153 patients in an international database. Arthritis Rheum. 2006;54(12):3971–8.
La Torre F, Martini G, Russo, et al. A preliminary disease severity score for juvenile systemic sclerosis. Arthritis Rheum. 2012;64(12):4143–50.
van den Hoogen FH, Boerbooms AM, Swaak AJ, et al. Comparison of methotrexate with placebo in the treatment of systemic sclerosis: a 24 week randomized double-blind trial, followed by a 24 week observational trial. Br J Rheumatol. 1996;35:364–72.
Pope JE, Bellamy N, Seibold JR, Baron M, Ellman M, Carette S, et al. A randomized, controlled trial of methotrexate versus placebo in early diffuse scleroderma. Arthritis Rheum. 2001;44(6):1351–8.
•• Kowal-Bielecka O, Fransen J, Avouac J, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum dis. 2017;76(8):1327–39. https://doi.org/10.1136/annrheumdis-2016-209909Updated EULAR recommendations for the treatment of systemic sclerosis. A roadmap for addressing the treatment also for pediatric-onset systemic sclerosis.
Omair MA, Alahmadi A, Johnson SR. Safety and effectiveness of mycophenolate in systemic sclerosis. A systematic review. PLoS One. 2015;10(5):e0124205. https://doi.org/10.1371/journal.pone.0124205.
Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4(9):708–19. https://doi.org/10.1016/S2213-2600(16)30152-7.
• Stevens AM, Torok KS, Li SC, Taber SF, Lu TT, Zulian F. Immunopathogenesis of juvenile systemic sclerosis. Front Immunol. 2019;10:1352. https://doi.org/10.3389/fimmu.2019.01352Review on recent findings on the immunopathogenesis of juvenile systemic sclerosis showing that early-onset SSc is a distinct entity and underlying the need for more extensive genetic studies in families with disease onset in early infancy.
Allanore Y, Simms R, Distler O, Trojanowska M, Pope J, Denton CP, et al. Systemic sclerosis. Nat Rev Dis Primers. 2015;1:15002. https://doi.org/10.1038/nrdp.2015.2.
O'Reilly S, Cant R, Ciechomska M, van Laar JM. Interleukin-6: a new therapeutic target in systemic sclerosis? Clin Transl Immunology. 2013;2(4):e4. https://doi.org/10.1038/cti.2013.2.
Khanna D, Denton CP, Jahreis A, van Laar JM, Frech TM, Anderson ME, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet. 2016;387(10038):2630–40. https://doi.org/10.1016/S0140-6736(16)00232-4.
• Khanna D, Spino C, Johnson S, et al. Abatacept in early diffuse cutaneous systemic sclerosis: results of a phase ii investigator-initiated, multicenter, double-blind, randomized, placebo-controlled trial. Arthritis Rheumatol. 2020;72(1):125–36. https://doi.org/10.1002/art.41055Study on a phase II trial showing that Abatacept improved skin involvement although not statistically significant. Secondary outcome measures, including gene expression subsets, showed evidence in support of abatacept.
Kraaij MD, van Laar JM. The role of B cells in systemic sclerosis. Biologics. 2008;2(3):389–95.
• Daoussis D, Melissaropoulos K, Sakellaropoulose G, et al. A multicenter, open-label, comparative study of B-cell depletion therapy with rituximab for systemic sclerosis-associated interstitial lung disease. Sem Arthritis Rheum. 2017;46:625–31. https://doi.org/10.1016/j.semarthrit.2016.10.003Open-label non RCT showing that rituximab improved skin and pulmonary involvement in adult patients with systemic sclerosis.
Jordan S, Distler JH, Maurer B, et al. Effects and safety of rituximab in systemic sclerosis: an analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann Rheum Dis. 2015;74(6):1188–94. https://doi.org/10.1136/annrheumdis-2013-204522.
Bosello SL, De Luca G, Rucco M, et al. Long-term efficacy of B cell depletion therapy on lung and skin involvement in diffuse systemic sclerosis. Semin Arthritis Rheum. 2014;44:428–36. https://doi.org/10.1016/j.semarthrit.2014.09.002.
•• Elhai M, Boubaya M, Distler O, et al. Outcomes of patients with systemic sclerosis treated with rituximab in contemporary practice: a prospective cohort study. Ann Rheum dis. 2019;78(7):979–87. https://doi.org/10.1136/annrheumdis-2018-214816Large prospective observational study comparing rituximab with standard treatment in a large cohort of SSc patients from the Eustar database. Rituximab showed a safe profile and improved skin and pulmonary involvement when associate with a concomitant MMF treatment.
Saunders P, Tsipouri V, Keir GJ, Ashby D, Flather MD, Parfrey H, et al. Rituximab versus cyclophosphamide for the treatment of connective tissue disease-associated interstitial lung disease (RECITAL): study protocol for a randomised controlled trial. Trials. 2017;18(1):275. https://doi.org/10.1186/s13063-017-2016-2.
Zulian F, Dal Pozzolo R, Meneghel A, et al. Rituximab for rapidly progressive juvenile systemic sclerosis. Rheumatology (Oxford). 2020; keaa193. https://doi.org/10.1093/rheumatology/keaa193.
Iwamoto N, Distler JH, Distler O. Tyrosine kinase inhibitors in the treatment of systemic sclerosis: from animal models to clinical trials. Curr Rheumatol Rep. 2011;13(1):21–7. https://doi.org/10.1007/s11926-010-0142-x.
Spiera RF, Gordon JK, Mersten JN, Magro CM, Mehta M, Wildman HF, et al. Imatinib mesylate (Gleevec) in the treatment of diffuse cutaneous systemic sclerosis: results of a 1-year, phase IIa, single-arm. Open-Label Clinical Trial Ann Rheum Dis. 2011;70(6):1003–9. https://doi.org/10.1136/ard.2010.143974.
Prey S, Ezzedine K, Doussau A, Grandoulier AS, Barcat D, Chatelus E, et al. Imatinib mesylate in scleroderma-associated diffuse skin fibrosis: a phase II multicentre randomized double-blinded controlled trial. Br J Dermatol. 2012;167(5):1138–44. https://doi.org/10.1111/j.1365-2133.2012.11186.x.
• Distler O, Highland KB, Gahlemann M, et al. Nintedanib for Systemic Sclerosis–Associated Interstitial Lung Disease. N Engl J Med. 2019;381(16):1596–7. https://doi.org/10.1056/NEJMc1910735Recent randomized control trial showing that nintedanib, a tyrosinkinase inhibitor, was associated with significantly lower deterioration of lung function, especially if associated with MMF. Unfortunately, no relevant effects on other organ involvement, such as skin thickness, were demonstrated.
Dees C, Tomcik M, Palumbo-Zerr K, Distler A, Beyer C, Lang V, et al. JAK-2 as a novel mediator of the profibrotic effects of transforming growth factor-β in systemic sclerosis. Arthritis Rheum. 2012;64(9):3006–15. https://doi.org/10.1002/art.34500.
Martini A, Maccario R, Ravelli A, Montagna D, de Benedetti F, Bonetti F, et al. Marked and sustained improvement two years after autologous stem cell transplantation in a girl with systemic sclerosis. Arthritis Rheum. 1999;42(4):807–11. https://doi.org/10.1002/1529-0131(199904)42:4<807::AID-ANR26>3.0.CO;2-T.
van Laar JM, Farge D, Sont JK, Naraghi K, Marjanovic Z, Larghero J, et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA. 2014;311(24):2490–8. https://doi.org/10.1001/jama.2014.6368.
•• Sullivan KM, Goldmuntz EA, Keyes-Elstein L, et al. Myeloablative Autologous Stem-Cell transplantation for Severe Scleroderma. N Engl J Med. 2018;378(1):35–47. https://doi.org/10.1056/nejmoa1703327Result of an open-label randomized trial comparing hematopoietic stem cell transplantation (HSCT) with CYC treatment in adult patients with severe SSc. A prolonged overall survival and organ failure-free survival was observed in HSCT group. Indeed, no transplant-related deaths have been observed during the first 12 months.
Hashkes PJ, Becker ML, Cabral DA, Laxer RM, Paller AS, Rabinovich CE, et al. Methotrexate: new uses for an old drug. J Pediatr. 2014;164(2):231–6. https://doi.org/10.1016/j.jpeds.2013.10.029.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pediatric Rheumatology
Rights and permissions
About this article
Cite this article
Zulian, F., Tirelli, F. Treatment in Juvenile Scleroderma. Curr Rheumatol Rep 22, 45 (2020). https://doi.org/10.1007/s11926-020-00910-x
Published:
DOI: https://doi.org/10.1007/s11926-020-00910-x