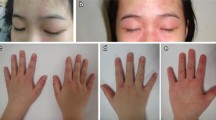
Abstract
Purpose of Review
We seek to provide a summary of the recent advances in juvenile dermatomyositis (JDM) that are moving the field toward precision care and personalized medicine for this uncommon condition.
Recent Findings
There has been a recent international focus on developing uniform classification, disease monitoring, and treatment for juvenile dermatomyositis. In addition, there has been a steady development of translational studies to determine the genetic determinants, transcriptomic profiles, and immune cell phenotypes in JDM.
Summary
Recent work toward standardization of disease classification, monitoring, and assessments together with advances in science, technology, and computing will facilitate the advancement toward true precision and personalized medicine in juvenile dermatomyositis in the near future.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rider LG, Nistala K. The juvenile idiopathic inflammatory myopathies: pathogenesis, clinical and autoantibody phenotypes, and outcomes. J Intern Med. 2016. https://doi.org/10.1111/joim.12444.
Pachman LM, Khojah AM. Advances in juvenile dermatomyositis: myositis specific antibodies aid in understanding disease heterogeneity. J Pediatr. 2018;195:16–27. https://doi.org/10.1016/j.jpeds.2017.12.053.
Huber AM, Mamyrova G, Lachenbruch PA, et al. Early illness features associated with mortality in the juvenile idiopathic inflammatory myopathies. Arthritis Care Res. 2014;66(5):732–40. https://doi.org/10.1002/acr.22212.
Mathiesen P, Hegaard H, Herlin T, Zak M, Pedersen FK, Nielsen S. Long-term outcome in patients with juvenile dermatomyositis: a cross-sectional follow-up study. Scand J Rheumatol. 2012. https://doi.org/10.3109/03009742.2011.608376.
Rider LG, Shah M, Mamyrova G, et al. The myositis autoantibody phenotypes of the juvenile idiopathic inflammatory myopathies. Medicine (Baltimore). 2013;92(4):223–43. https://doi.org/10.1097/MD.0b013e31829d08f9.
Okong’o LO, Esser M, Wilmshurst J, Scott C. Characteristics and outcome of children with juvenile dermatomyositis in Cape Town: a cross-sectional study. Pediatr Rheumatol Online J. 2016;14(1):60. https://doi.org/10.1186/s12969-016-0118-0.
Tsaltskan V, Aldous A, Serafi S, et al. Long-term outcomes in Juvenile Myositis patients. Semin Arthritis Rheum. June 2019. https://doi.org/10.1016/j.semarthrit.2019.06.014.
Mamyrova G, Rider LG, Ehrlich A, et al. Environmental factors associated with disease flare in juvenile and adult dermatomyositis. Rheumatology (Oxford). 2017;56(8):1342–7. https://doi.org/10.1093/rheumatology/kex162.
Habers GEA, Huber AM, Mamyrova G, et al. Brief report: association of myositis autoantibodies, clinical features, and environmental exposures at illness onset with disease course in juvenile myositis. Arthritis Rheum. 2016;68(3):761–8. https://doi.org/10.1002/art.39466.
Orandi AB, Dharnidharka VR, Al-Hammadi N, Baszis KW. Clinical phenotypes and biologic treatment use in juvenile dermatomyositis-associated calcinosis. Pediatr Rheumatol. 2018;16(1):84. https://doi.org/10.1186/s12969-018-0299-9.
Phillippi K, Hoeltzel M, Byun Robinson A, et al. Race, Income, and Disease Outcomes in Juvenile Dermatomyositis. J Pediatr. 2017;184:38–44. https://doi.org/10.1016/j.jpeds.2017.01.046.
Neely J, Long CS, Sturrock H, Kim S. The association of short-term ultraviolet radiation exposure and disease severity in juvenile dermatomyositis. Arthritis Care Res. 2019. https://doi.org/10.1002/acr.23840.
Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med. 1975;292(8):403–7. https://doi.org/10.1056/NEJM197502202920807.
•• Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis. 2017;76(12):1955–64. https://doi.org/10.1136/annrheumdis-2017-211468. This article outlines new classification criteria for idiopathic inflammatory myopathies, including JDM, replacing the previously used Bohan and Peter criteria developed in 1975.
Zhang X, Yang X, Ji L, Zhang Z. Validation of 2017 classification criteria for adult and juvenile idiopathic inflammatory myopathies proposed by EULAR/ACR in Chinese patients. Int J Rheum Dis. 2019. https://doi.org/10.1111/1756-185X.13605.
Rider LG, Aggarwal R, Pistorio A, et al. 2016 American College of Rheumatology/European League Against Rheumatism criteria for minimal, moderate, and major clinical response in juvenile dermatomyositis: an International Myositis Assessment and Clinical Studies Group/Paediatric Rheumatology Inter. Arthritis Rheum. 2017;69(5):911–23. https://doi.org/10.1002/art.40060.
Rider LG, Werth VP, Huber AM, et al. Measures of adult and juvenile dermatomyositis, polymyositis, and inclusion body myositis: Physician and Patient/Parent Global Activity, Manual Muscle Testing (MMT), Health Assessment Questionnaire (HAQ)/Childhood Health Assessment Questionnaire (C-HAQ). Arthritis Care Res. 2011;63(S11):S118–57. https://doi.org/10.1002/acr.20532.
Rider LG, Miller FW, Feldman BM, et al. Development of validated disease activity and damage indices for the juvenile idiopathic inflammatory myopathies. I. Physician, parent, and patient global assessments. Arthritis Rheum. 1997;40(11):1976–83. https://doi.org/10.1002/art.1780401109.
Ruperto N, Ravelli A, Pistorio A, et al. The provisional Paediatric Rheumatology International Trials Organisation/American College of Rheumatology/European League Against Rheumatism Disease activity core set for the evaluation of response to therapy in juvenile dermatomyositis: a prospective validation study. Arthritis Rheum. 2008;59(1):4–13. https://doi.org/10.1002/art.23248.
McCann LJ, Kirkham JJ, Wedderburn LR, et al. Development of an internationally agreed minimal dataset for juvenile dermatomyositis (JDM) for clinical and research use. Trials. 2015;16(1):268. https://doi.org/10.1186/s13063-015-0784-0.
Rosina S, Consolaro A, van Dijkhuizen P, et al. Development and validation of a composite disease activity score for measurement of muscle and skin involvement in juvenile dermatomyositis. Rheumatology. 2019;58(7):1196–205. https://doi.org/10.1093/rheumatology/key421.
Varnier GC, Rosina S, Ferrari C, et al. Development and testing of a hybrid measure of muscle strength in juvenile dermatomyositis for use in routine care. Arthritis Care Res. 2018;70(9):1312–9. https://doi.org/10.1002/acr.23491.
Tiao J, Feng R, Berger EM, et al. Evaluation of the reliability of the Cutaneous Dermatomyositis Disease Area and Severity Index and the Cutaneous Assessment Tool-Binary Method in juvenile dermatomyositis among paediatric dermatologists, rheumatologists and neurologists. Br J Dermatol. 2017;177(4):1086–92. https://doi.org/10.1111/bjd.15596.
Enders FB, Bader-Meunier B, Baildam E, et al. Consensus-based recommendations for the management of juvenile dermatomyositis. Ann Rheum Dis. 2017;76(2):329–40. https://doi.org/10.1136/annrheumdis-2016-209247.
Ringold S, Nigrovic PA, Feldman BM, et al. The Childhood Arthritis and Rheumatology Research Alliance consensus treatment plans. Arthritis Rheum. 2018;70(5):669–78. https://doi.org/10.1002/art.40395.
Huber AM, Kim S, Reed AM, et al. Childhood arthritis and rheumatology research alliance consensus clinical treatment plans for juvenile dermatomyositis with persistent skin rash. J Rheumatol. 2017;44(1):110–6. https://doi.org/10.3899/jrheum.160688
Kim S, Kahn P, Robinson AB, et al. Childhood Arthritis and Rheumatology Research Alliance consensus clinical treatment plans for juvenile dermatomyositis with skin predominant disease. Pediatr Rheumatol. 2017;15(1):1. https://doi.org/10.1186/s12969-016-0134-0.
Hinze CH, Oommen PT, Dressler F, et al. Development of practice and consensus-based strategies including a treat-to-target approach for the management of moderate and severe juvenile dermatomyositis in Germany and Austria. Pediatr Rheumatol. 2018;16(1):40. https://doi.org/10.1186/s12969-018-0257-6.
Tansley SL, Betteridge ZE, Gunawardena H, et al. Anti-MDA5 autoantibodies in juvenile dermatomyositis identify a distinct clinical phenotype: a prospective cohort study. Arthritis Res Ther. 2014;16(4):R138. https://doi.org/10.1186/ar4600.
Sabbagh S, Pinal-Fernandez I, Kishi T, et al. Anti-Ro52 autoantibodies are associated with interstitial lung disease and more severe disease in patients with juvenile myositis. Ann Rheum Dis. 2019;78(7):988–95. https://doi.org/10.1136/ANNRHEUMDIS-2018-215004.
Miller FW, Chen W, O’Hanlon TP, et al. Genome-wide association study identifies HLA 8.1 ancestral haplotype alleles as major genetic risk factors for myositis phenotypes. Genes Immun. 2015;16(7):470–80. https://doi.org/10.1038/gene.2015.28.
•• Rothwell S, Cooper RG, Lundberg IE, et al. Dense genotyping of immune-related loci in idiopathic inflammatory myopathies confirms HLA alleles as the strongest genetic risk factor and suggests different genetic background for major clinical subgroups. Ann Rheum Dis. 2016;75(8):1558–66. https://doi.org/10.1136/ANNRHEUMDIS-2015-208119. This is the largest GWAS of idiopathic inflammatory myopathies to date identifying specific immune genes associated with the clinical subtypes of disease.
Lintner KE, Patwardhan A, Rider LG, et al. Gene copy-number variations (CNVs) of complement C4 and C4A deficiency in genetic risk and pathogenesis of juvenile dermatomyositis. Ann Rheum Dis. 2016;75(9):1599–606. https://doi.org/10.1136/annrheumdis-2015-207762.
Moneta GM, Pires Marafon D, Marasco E, et al. Muscle expression of type I and type II interferons is increased in juvenile dermatomyositis and related to clinical and histological features. Arthritis Rheumatol. 2018. https://doi.org/10.1002/art.40800.
Neely J, Rychkov D, Paranjpe M, Waterfield M, Kim S, Sirota M. Gene Expression Meta‐Analysis Reveals Concordance in Gene Activation, Pathway, and Cell‐Type Enrichment in Dermatomyositis Target Tissues. ACR Open Rheumatol. 2019. https://doi.org/10.1002/acr2.11081.
Gitiaux C, Latroche C, Weiss-Gayet M, et al. Myogenic progenitor cells exhibit type I interferon-driven proangiogenic properties and molecular signature during juvenile dermatomyositis. Arthritis Rheum. 2018;70(1):134–45. https://doi.org/10.1002/art.40328.
Throm AA, Alinger JB, Pingel JT, Daugherty AL, Pachman LM, French AR. Dysregulated NK cell PLCγ2 signaling and activity in juvenile dermatomyositis. JCI Insight. 2018;3(22). https://doi.org/10.1172/jci.insight.123236.
Fasano S, Gordon P, Hajji R, Loyo E, Isenberg DA. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2017;56(1):26–36. https://doi.org/10.1093/rheumatology/kew146.
Piper CJM, Wilkinson MGL, Deakin CT, et al. CD19+CD24hiCD38hi B cells are expanded in juvenile dermatomyositis and exhibit a pro-inflammatory phenotype after activation through toll-like receptor 7 and interferon-α. Front Immunol. 2018;9:1372. https://doi.org/10.3389/fimmu.2018.01372.
Olazagasti JM, Hein M, Crowson CS, et al. Adipokine gene expression in peripheral blood of adult and juvenile dermatomyositis patients and their relation to clinical parameters and disease activity measures. J Inflamm. 2015;12(1):29. https://doi.org/10.1186/s12950-015-0075-2.
Crowson CS, Hein MS, Pendegraft RS, et al. Interferon chemokine score and other cytokine measures track with changes in disease activity in patients with juvenile and adult dermatomyositis. ACR Open Rheumatol. 2019;1(2):83–9. https://doi.org/10.1002/acr2.1011.
Huard C, Gullà SV, Bennett DV, Coyle AJ, Vleugels RA, Greenberg SA. Correlation of cutaneous disease activity with type 1 interferon gene signature and interferon β in dermatomyositis. Br J Dermatol. 2017;176(5):1224–30. https://doi.org/10.1111/bjd.15006.
•• Bellutti Enders F, van Wijk F, Scholman R, et al. Correlation of CXCL10, tumor necrosis factor receptor type II, and galectin 9 with disease activity in juvenile dermatomyositis. Arthritis Rheum. 2014;66(8):2281–9. https://doi.org/10.1002/art.38676.. This is the largest study of disease biomarkers for JDM validated in an international cohort.
Wienke J, Bellutti Enders F, Lim J, et al. Galectin-9 and CXCL10 as biomarkers for disease activity in juvenile dermatomyositis: a longitudinal cohort study and multicohort validation. Arthritis Rheum. 2019;71(8):1377–90. https://doi.org/10.1002/art.40881.
Mamyrova G, Kishi T, Targoff IN, et al. Features distinguishing clinically amyopathic juvenile dermatomyositis from juvenile dermatomyositis. Rheumatology. 2018;57(11):1956–63. https://doi.org/10.1093/rheumatology/key190.
Ruperto N, Pistorio A, Oliveira S, et al. Prednisone versus prednisone plus ciclosporin versus prednisone plus methotrexate in new-onset juvenile dermatomyositis: a randomised trial. Lancet. 2016;387(10019):671–8. https://doi.org/10.1016/S0140-6736(15)01021-1.
Giancane G, Lavarello C, Pistorio A, et al. The PRINTO evidence-based proposal for glucocorticoids tapering/discontinuation in new onset juvenile dermatomyositis patients. Pediatr Rheumatol. 2019;17(1):24. https://doi.org/10.1186/s12969-019-0326-5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pediatric Rheumatology
Rights and permissions
About this article
Cite this article
Neely, J., Kim, S. Advances Toward Precision Medicine in Juvenile Dermatomyositis. Curr Rheumatol Rep 21, 73 (2019). https://doi.org/10.1007/s11926-019-0873-2
Published:
DOI: https://doi.org/10.1007/s11926-019-0873-2