Abstract
Purpose of Review
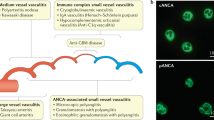
The ANCA-associated vasculitides are a group of small vessel vasculitides characterized by autoantibodies recognizing the neutrophil cytoplasmic antigens PR3 and MPO. We examine the current clinical and molecular immunology understanding of ANCA-associated vasculitides and discuss the current needs in our understanding of the pathogenic mechanisms of these rare diseases.
Recent Findings
The majority of efforts to understand the pathogenesis of these diseases have focused on dissecting neutrophil biology because the neutrophil is the primary expressor of ANCA autoantigens. However, a number of important genetic, clinical, and cellular biology observations suggest that attempts to understand the pathogenesis of ANCA vasculitides should move away from emphasis on the role of the neutrophil and instead re-focus on the potential role of other immune cell mediators.
Summary
Whether or not neutrophils are the key determinant of ANCA-associated vasculitis pathogenesis should be revisited in detail. A neutrophil-centric view of the pathogenesis of these diseases cannot fully account for important genetic, clinical, and cellular biology observations that implicate important and under-appreciated roles for monocytes and T cells. Refocusing on these findings will likely lead to new discovery of novel therapeutic targets and the identification of clinically useful biomarkers for disease activity.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65(1):1–11. doi:10.1002/art.37715.
Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116(6):488–98.
Stone JH, Wegener’s Granulomatosis Etanercept Trial Research G. Limited versus severe Wegener’s granulomatosis: baseline data on patients in the Wegener’s granulomatosis etanercept trial. Arthritis Rheum. 2003;48(8):2299–309. doi:10.1002/art.11075.
Guillevin L, Durand-Gasselin B, Cevallos R, Gayraud M, Lhote F, Callard P, et al. Microscopic polyangiitis: clinical and laboratory findings in eighty-five patients. Arthritis Rheum. 1999;42(3):421–30. doi:10.1002/1529-0131(199904)42:3<421::AID-ANR5>3.0.CO;2-6.
Langford C. Clinical features and diagnosis of small-vessel vasculitis. Cleve Clin J Med. 2012;79 Suppl 3:S3–7. doi:10.3949/ccjm.79.s3.01.
Ahn JK, Hwang JW, Lee J, Jeon CH, Cha HS, Koh EM. Clinical features and outcome of microscopic polyangiitis under a new consensus algorithm of ANCA-associated vasculitides in Korea. Rheumatol Int. 2012;32(10):2979–86. doi:10.1007/s00296-011-2079-4.
Keogh KA, Specks U. Churg-Strauss syndrome: clinical presentation, antineutrophil cytoplasmic antibodies, and leukotriene receptor antagonists. Am J Med. 2003;115(4):284–90.
Comarmond C, Pagnoux C, Khellaf M, Cordier JF, Hamidou M, Viallard JF, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. 2013;65(1):270–81. doi:10.1002/art.37721.
Davies DJ, Moran JE, Niall JF, Ryan GB. Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? Br Med J (Clin Res Ed). 1982;285(6342):606.
Hall JB, Wadham BM, Wood CJ, Ashton V, Adam WR. Vasculitis and glomerulonephritis: a subgroup with an antineutrophil cytoplasmic antibody. Aust N Z J Med. 1984;14(3):277–8.
van der Woude FJ, Rasmussen N, Lobatto S, Wiik A, Permin H, van Es LA, et al. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener’s granulomatosis. Lancet. 1985;1(8426):425–9.
Hoffman GS, Specks U. Antineutrophil cytoplasmic antibodies. Arthritis Rheum. 1998;41(9):1521–37. doi:10.1002/1529-0131(199809)41:9<1521::AID-ART2>3.0.CO;2-A.
Wiik A. What you should know about PR3-ANCA. An introduction. Arthritis Res. 2000;2(4):252–4. doi:10.1186/ar96.
Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110(7):955–63. doi:10.1172/JCI15918.
Schlieben DJ, Korbet SM, Kimura RE, Schwartz MM, Lewis EJ. Pulmonary-renal syndrome in a newborn with placental transmission of ANCAs. Am J Kidney Dis. 2005;45(4):758–61.
Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci U S A. 1990;87(11):4115–9.
Porges AJ, Redecha PB, Kimberly WT, Csernok E, Gross WL, Kimberly RP. Anti-neutrophil cytoplasmic antibodies engage and activate human neutrophils via Fc gamma RIIa. J Immunol. 1994;153(3):1271–80.
Kocher M, Siegel ME, Edberg JC, Kimberly RP. Cross-linking of Fc gamma receptor IIa and Fc gamma receptor IIIb induces different proadhesive phenotypes on human neutrophils. J Immunol. 1997;159(8):3940–8.
Williams JM, Ben-Smith A, Hewins P, Dove SK, Hughes P, McEwan R, et al. Activation of the G(i) heterotrimeric G protein by ANCA IgG F(ab’)2 fragments is necessary but not sufficient to stimulate the recruitment of those downstream mediators used by intact ANCA IgG. J Am Soc Nephrol. 2003;14(3):661–9.
Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221–32. doi:10.1056/NEJMoa0909905.
Jones RB, Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363(3):211–20. doi:10.1056/NEJMoa0909169.
Specks U, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, et al. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med. 2013;369(5):417–27. doi:10.1056/NEJMoa1213277.
• Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaitre O, Cohen P, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014;371(19):1771–80. doi:10.1056/NEJMoa1404231. This trial demonstrated the efficacy of B cell depletion with Rituximab in maintenance treatment of AAV.
Tadema H, Heeringa P, Kallenberg CG. Bacterial infections in Wegener’s granulomatosis: mechanisms potentially involved in autoimmune pathogenesis. Curr Opin Rheumatol. 2011;23(4):366–71. doi:10.1097/BOR.0b013e328346c332.
Jennette JC, Falk RJ. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat Rev Rheumatol. 2014;10(8):463–73. doi:10.1038/nrrheum.2014.103.
Jennette JC, Falk RJ, Gasim AH. Pathogenesis of antineutrophil cytoplasmic autoantibody vasculitis. Curr Opin Nephrol Hypertens. 2011;20(3):263–70. doi:10.1097/MNH.0b013e3283456731.
Pendergraft 3rd WF, Preston GA, Shah RR, Tropsha A, Carter Jr CW, Jennette JC, et al. Autoimmunity is triggered by cPR-3(105-201), a protein complementary to human autoantigen proteinase-3. Nat Med. 2004;10(1):72–9. doi:10.1038/nm968.
Yang J, Bautz DJ, Lionaki S, Hogan SL, Chin H, Tisch RM, et al. ANCA patients have T cells responsive to complementary PR-3 antigen. Kidney Int. 2008;74(9):1159–69. doi:10.1038/ki.2008.309.
Stegeman CA, Tervaert JW, Sluiter WJ, Manson WL, de Jong PE, Kallenberg CG. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann Intern Med. 1994;120(1):12–7.
Zycinska K, Wardyn KA, Zielonka TM, Demkow U, Traburzynski MS. Chronic crusting, nasal carriage of Staphylococcus aureus and relapse rate in pulmonary Wegener’s granulomatosis. J Physiol Pharmacol. 2008;59 Suppl 6:825–31.
Laudien M, Gadola SD, Podschun R, Hedderich J, Paulsen J, Reinhold-Keller E, et al. Nasal carriage of Staphylococcus aureus and endonasal activity in Wegener s granulomatosis as compared to rheumatoid arthritis and chronic Rhinosinusitis with nasal polyps. Clin Exp Rheumatol. 2010;28(1 Suppl 57):51–5.
Stegeman CA, Tervaert JW, de Jong PE, Kallenberg CG. Trimethoprim-sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener’s granulomatosis. Dutch Co-Trimoxazole Wegener Study Group. N Engl J Med. 1996;335(1):16–20. doi:10.1056/NEJM199607043350103.
Kain R, Exner M, Brandes R, Ziebermayr R, Cunningham D, Alderson CA, et al. Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med. 2008;14(10):1088–96. doi:10.1038/nm.1874.
Roth AJ, Brown MC, Smith RN, Badhwar AK, Parente O, Chung H, et al. Anti-LAMP-2 antibodies are not prevalent in patients with antineutrophil cytoplasmic autoantibody glomerulonephritis. J Am Soc Nephrol. 2012;23(3):545–55. doi:10.1681/ASN.2011030273.
McInnis EA, Badhwar AK, Muthigi A, Lardinois OM, Allred SC, Yang J, et al. Dysregulation of autoantigen genes in ANCA-associated vasculitis involves alternative transcripts and new protein synthesis. J Am Soc Nephrol. 2015;26(2):390–9. doi:10.1681/ASN.2013101092.
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. doi:10.1126/science.1092385.
Sangaletti S, Tripodo C, Chiodoni C, Guarnotta C, Cappetti B, Casalini P, et al. Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood. 2012;120(15):3007–18. doi:10.1182/blood-2012-03-416156.
Weidner S, Carl M, Riess R, Rupprecht HD. Histologic analysis of renal leukocyte infiltration in antineutrophil cytoplasmic antibody-associated vasculitis: importance of monocyte and neutrophil infiltration in tissue damage. Arthritis Rheum. 2004;50(11):3651–7. doi:10.1002/art.20607.
Schonermarck U, Csernok E, Trabandt A, Hansen H, Gross WL. Circulating cytokines and soluble CD23, CD26 and CD30 in ANCA-associated vasculitides. Clin Exp Rheumatol. 2000;18(4):457–63.
Wang G, Hansen H, Tatsis E, Csernok E, Lemke H, Gross WL. High plasma levels of the soluble form of CD30 activation molecule reflect disease activity in patients with Wegener’s granulomatosis. Am J Med. 1997;102(6):517–23.
Abdulahad WH, Stegeman CA, van der Geld YM, Doornbos-van der Meer B, Limburg PC, Kallenberg CG. Functional defect of circulating regulatory CD4+ T cells in patients with Wegener’s granulomatosis in remission. Arthritis Rheum. 2007;56(6):2080–91. doi:10.1002/art.22692.
Rimbert M, Hamidou M, Braudeau C, Puechal X, Teixeira L, Caillon H, et al. Decreased numbers of blood dendritic cells and defective function of regulatory T cells in antineutrophil cytoplasmic antibody-associated vasculitis. PLoS One. 2011;6(4):e18734. doi:10.1371/journal.pone.0018734.
• Free ME, Bunch DO, McGregor JA, Jones BE, Berg EA, Hogan SL, et al. Patients with antineutrophil cytoplasmic antibody-associated vasculitis have defective Treg cell function exacerbated by the presence of a suppression-resistant effector cell population. Arthritis Rheum. 2013;65(7):1922–33. doi:10.1002/art.37959. Analysis of peripheral blood samples from AAV patients demonstrated the presence of impaired suppressor function in T reg cells, as well as the presence of a proinflammatory CD4+ T cell population.
Ooi JD, Chang J, Hickey MJ, Borza DB, Fugger L, Holdsworth SR, et al. The immunodominant myeloperoxidase T-cell epitope induces local cell-mediated injury in antimyeloperoxidase glomerulonephritis. Proc Natl Acad Sci U S A. 2012;109(39):E2615–24. doi:10.1073/pnas.1210147109.
•• Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, Jayne DR, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. 2012;367(3):214–23. doi:10.1056/NEJMoa1108735. This GWAS study performed in European AAV patients demonstrated distinct MHC associations in GPA versus MPA. Mutations in the gene encoding PR3 (PRTN3) were also linked to the presence of anti-PR3 antibodies.
Xie G, Roshandel D, Sherva R, Monach PA, Lu EY, Kung T, et al. Association of granulomatosis with polyangiitis (Wegener’s) with HLA-DPB1*04 and SEMA6A gene variants: evidence from genome-wide analysis. Arthritis Rheum. 2013;65(9):2457–68. doi:10.1002/art.38036.
•• Merkel PA, Xie G, Monach PA, Ji X, Ciavatta DJ, Byun J, et al. Identification of functional and expression polymorphisms associated with risk for anti-neutrophil cytoplasmic autoantibody-associated vasculitis. Arthritis Rheumatol. 2016. doi:10.1002/art.40034. This most recent genome-wide association study confirmed the reports of prior studies that specific MHC class II alleles are associated with increased risk for developing both granulomatosis with polyangiitis and microscopic polyangiitis.
Unanue ER, Turk V, Neefjes J. Variations in MHC class II antigen processing and presentation in health and disease. Annu Rev Immunol. 2016;34:265–97. doi:10.1146/annurev-immunol-041015-055420.
Granados DP, Sriranganadane D, Daouda T, Zieger A, Laumont CM, Caron-Lizotte O, et al. Impact of genomic polymorphisms on the repertoire of human MHC class I-associated peptides. Nat Commun. 2014;5:3600. doi:10.1038/ncomms4600.
Jennette JC, Falk RJ, Hu P, Xiao H. Pathogenesis of antineutrophil cytoplasmic autoantibody-associated small-vessel vasculitis. Annu Rev Pathol. 2013;8:139–60. doi:10.1146/annurev-pathol-011811-132453.
Cheadle C, Berger AE, Andrade F, James R, Johnson K, Watkins T, et al. Transcription of proteinase 3 and related myelopoiesis genes in peripheral blood mononuclear cells of patients with active Wegener’s granulomatosis. Arthritis Rheum. 2010;62(6):1744–54. doi:10.1002/art.27398.
•• O’Brien EC, Abdulahad WH, Rutgers A, Huitema MG, O’Reilly VP, Coughlan AM, et al. Intermediate monocytes in ANCA vasculitis: increased surface expression of ANCA autoantigens and IL-1beta secretion in response to anti-MPO antibodies. Sci Rep. 2015;5:11888. doi:10.1038/srep11888. This study identified a subset of circulating monocytes which are increased in AAV patients and express high levels of MPO and PR3. Anti-MPO antibodies stimulated the production of inflammatory cytokines in these monocytes.
Ralston DR, Marsh CB, Lowe MP, Wewers MD. Antineutrophil cytoplasmic antibodies induce monocyte IL-8 release. Role of surface proteinase-3, alpha1-antitrypsin, and Fcgamma receptors. J Clin Invest. 1997;100(6):1416–24. doi:10.1172/JCI119662.
Acknowledgements
The work reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number T32AR048522 and by National Institutes of Health Award Number RO1 DE 12354-15A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Gapud is supported by funding from the National Institutes of Health Award Number T32AR048522. Dr. Antiochos is supported by the Jerome L. Greene Foundation and National Institutes of Health Award Number RO1 DE 12354-15A1. Dr. Seo was supported by the Dr. Darwin James Liao Discovery Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Vasculitis
Rights and permissions
About this article
Cite this article
Gapud, E.J., Seo, P. & Antiochos, B. ANCA-Associated Vasculitis Pathogenesis: A Commentary. Curr Rheumatol Rep 19, 15 (2017). https://doi.org/10.1007/s11926-017-0641-0
Published:
DOI: https://doi.org/10.1007/s11926-017-0641-0