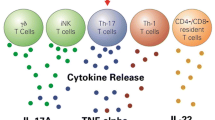
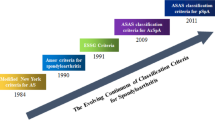
Abstract
Purpose of the Review
Progressive ankylosis is a feared consequence of long-standing axial spondyloarthritis. We aim to critically review current insights into the effect of therapy, the molecular pathways involved in this process, and to present a model explaining the sequence of events.
Recent Findings
Long-term follow-up data suggest that successful control of inflammation may slow down radiographic progression of disease in axial spondyloarthritis. Structural effects of new therapies such as interleukin-17 targeting need to be further studied. Bone loss and architectural changes could act as driver for the tissue remodeling process trying to maintain spinal stability in the presence of inflammation.
Summary
Despite some progress, the nature and mechanisms of new bone formation in axial spondyloarthritis still remain incompletely understood. However, long-term control of inflammation appears critical to avoid progressive disability due to structural damage.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med. 2016;374(26):2563–74. doi:10.1056/NEJMra1406182.
Poddubnyy D, Rudwaleit M, Haibel H, Listing J, Marker-Hermann E, Zeidler H, et al. Rates and predictors of radiographic sacroiliitis progression over 2 years in patients with axial spondyloarthritis. Ann Rheum Dis. 2011;70(8):1369–74. doi:10.1136/ard.2010.145995.
Machado P, Landewe R, Braun J, Hermann KG, Baker D, van der Heijde D. Both structural damage and inflammation of the spine contribute to impairment of spinal mobility in patients with ankylosing spondylitis. Ann Rheum Dis. 2010;69(8):1465–70. doi:10.1136/ard.2009.124206.
• Callhoff J, Sieper J, Weiss A, Zink A, Listing J. Efficacy of TNFalpha blockers in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis: a meta-analysis. Ann Rheum Dis. 2015;74(6):1241–8. doi:10.1136/annrheumdis-2014-205322. This study provides an interesting summary on the use of TNF inhibitors in axial SpA.
Baeten D, Baraliakos X, Braun J, Sieper J, Emery P, van der Heijde D, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382(9906):1705–13. doi:10.1016/S0140-6736(13)61134-4.
Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373(26):2534–48. doi:10.1056/NEJMoa1505066.
Poddubnyy D, Hermann KG, Callhoff J, Listing J, Sieper J. Ustekinumab for the treatment of patients with active ankylosing spondylitis: results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann Rheum Dis. 2014;73(5):817–23. doi:10.1136/annrheumdis-2013-204248.
Lories RJ, Haroon N. Bone formation in axial spondyloarthritis. Best Pract Res Clin Rheumatol. 2014;28(5):765–77. doi:10.1016/j.berh.2014.10.008.
Maksymowych WP, Elewaut D, Schett G. Motion for debate: the development of ankylosis in ankylosing spondylitis is largely dependent on inflammation. Arthritis Rheum. 2012;64(6):1713–9. doi:10.1002/art.34442.
van der Heijde D, Landewe R, Baraliakos X, Houben H, van Tubergen A, Williamson P, et al. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum. 2008;58(10):3063–70. doi:10.1002/art.23901.
van der Heijde D, Landewe R, Einstein S, Ory P, Vosse D, Ni L, et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum. 2008;58(5):1324–31. doi:10.1002/art.23471.
van der Heijde D, Salonen D, Weissman BN, Landewe R, Maksymowych WP, Kupper H, et al. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther. 2009;11(4):R127. doi:10.1186/ar2794.
Haroon N, Inman RD, Learch TJ, Weisman MH, Lee M, Rahbar MH, et al. The impact of tumor necrosis factor alpha inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum. 2013;65(10):2645–54. doi:10.1002/art.38070.
Baraliakos X, Haibel H, Listing J, Sieper J, Braun J. Continuous long-term anti-TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitis. Ann Rheum Dis. 2014;73(4):710–5. doi:10.1136/annrheumdis-2012-202698.
• Maas F, Arends S, Brouwer E, Essers I, van der Veer E, Efde M et al. Reduction in spinal radiographic progression in ankylosing spondylitis patients receiving prolonged treatment with TNF-alpha inhibitors. Arthritis Care Res (Hoboken). 2016. doi:10.1002/acr.23097. Novel data about the potential effects of anti-TNF on radiographic progression.
Song IH, Hermann KG, Haibel H, Althoff CE, Poddubnyy D, Listing J, et al. Prevention of new osteitis on magnetic resonance imaging in patients with early axial spondyloarthritis during 3 years of continuous treatment with etanercept: data of the ESTHER trial. Rheumatology (Oxford). 2015;54(2):257–61. doi:10.1093/rheumatology/keu263.
Carter S, Lories RJ. Osteoporosis: a paradox in ankylosing spondylitis. Curr Osteoporos Rep. 2011;9(3):112–5. doi:10.1007/s11914-011-0058-z.
Wanders A, Heijde D, Landewe R, Behier JM, Calin A, Olivieri I, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 2005;52(6):1756–65. doi:10.1002/art.21054.
Poddubnyy D, Rudwaleit M, Haibel H, Listing J, Marker-Hermann E, Zeidler H, et al. Effect of non-steroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis. 2012;71(10):1616–22. doi:10.1136/annrheumdis-2011-201252.
•• Sieper J, Listing J, Poddubnyy D, Song IH, Hermann KG, Callhoff J, et al. Effect of continuous versus on-demand treatment of ankylosing spondylitis with diclofenac over 2 years on radiographic progression of the spine: results from a randomised multicentre trial (ENRADAS). Ann Rheum Dis. 2016;75(8):1438–43. doi:10.1136/annrheumdis-2015-207897. Important data about the difficulties to demonstrate a structure modifying effect of NSAID therapy.
Kroon F, Landewe R, Dougados M, van der Heijde D. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71(10):1623–9. doi:10.1136/annrheumdis-2012-201370.
Lories RJ, Schett G. Pathophysiology of new bone formation and ankylosis in spondyloarthritis. Rheum Dis Clin North Am. 2012;38(3):555–67. doi:10.1016/j.rdc.2012.08.003.
Maksymowych WP. Disease modification in ankylosing spondylitis. Nat Rev Rheumatol. 2010;6(2):75–81. doi:10.1038/nrrheum.2009.258.
Maksymowych WP, Morency N, Conner-Spady B, Lambert RG. Suppression of inflammation and effects on new bone formation in ankylosing spondylitis: evidence for a window of opportunity in disease modification. Ann Rheum Dis. 2013;72(1):23–8. doi:10.1136/annrheumdis-2011-200859.
Maksymowych WP, Wichuk S, Chiowchanwisawakit P, Lambert RG, Pedersen SJ. Fat metaplasia and backfill are key intermediaries in the development of sacroiliac joint ankylosis in patients with ankylosing spondylitis. Arthritis Rheumatol. 2014;66(11):2958–67. doi:10.1002/art.38792.
Chiowchanwisawakit P, Lambert RGW, Conner-Spady B, Maksymowych WP. Focal fat lesions at vertebral corners on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis. Arthritis Rheum-Us. 2011;63(8):2215–25. doi:10.1002/art.30393.
•• Machado PM, Baraliakos X, van der Heijde D, Braun J, Landewe R. MRI vertebral corner inflammation followed by fat deposition is the strongest contributor to the development of new bone at the same vertebral corner: a multilevel longitudinal analysis in patients with ankylosing spondylitis. Ann Rheum Dis. 2016;75(8):1486–93. doi:10.1136/annrheumdis-2015-208011. The paper provides good evidence for a sequential process leading to ankylosis.
Baraliakos X, Heldmann F, Callhoff J, Listing J, Appelboom T, Brandt J, et al. Which spinal lesions are associated with new bone formation in patients with ankylosing spondylitis treated with anti-TNF agents? A long-term observational study using MRI and conventional radiography. Ann Rheum Dis. 2014;73(10):1819–25. doi:10.1136/annrheumdis-2013-203425.
•• Baraliakos X, Boehm H, Samir A, Schett G, Braun J. Which cells correspond to typical signals for fatty and inflammatory lesions seen on MRI in AS? Clin Exp Rheumatol. 2016;34(4):744. New knowledge on the histological appearance of fatty lesions on MRI in SpA.
van der Heijde D, Machado P, Braun J, Hermann KGA, Baraliakos X, Hsu B, et al. MRI inflammation at the vertebral unit only marginally predicts new syndesmophyte formation: a multilevel analysis in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71(3):369–73. doi:10.1136/annrheumdis-2011-200208.
Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, et al. IL-23 induces spondyloarthropathy by acting on ROR-gammat+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. 2012;18(7):1069–76. doi:10.1038/nm.2817.
El-Sherbiny Y, Elzayadi A, Fragkakis EM, Cuthbert R, Baboolal T, Jones E et al. IL-22 drives the proliferation and differentiation of human bone marrow mesenchymal stem cells (MSCs); a novel pathway that may contribute to aberrant new bone formation in human spa and beyond. Arthritis & Rheumatology. 2015;67
Lefebvre V, Bhattaram P. Vertebrate skeletogenesis. Curr Top Dev Biol. 2010;90:291–317. doi:10.1016/S0070-2153(10)90008-2.
Lories RJ, Derese I, Luyten FP. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 2005;115(6):1571–9. doi:10.1172/JCI23738.
Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, et al. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13(2):156–63. doi:10.1038/nm1538.
Uderhardt S, Diarra D, Katzenbeisser J, David JP, Zwerina J, Richards W, et al. Blockade of Dickkopf (DKK)-1 induces fusion of sacroiliac joints. Ann Rheum Dis. 2010;69(3):592–7. doi:10.1136/ard.2008.102046.
Ruiz-Heiland G, Horn A, Zerr P, Hofstetter W, Baum W, Stock M, et al. Blockade of the hedgehog pathway inhibits osteophyte formation in arthritis. Ann Rheum Dis. 2012;71(3):400–7. doi:10.1136/ard.2010.148262.
Park MC, Park Y, Lee SK. Relationship of bone morphogenetic proteins to disease activity and radiographic damage in patients with ankylosing spondylitis. Scand J Rheumatol. 2008;37(3):200–4. doi:10.1080/03009740701774941.
Chen HA, Chen CH, Lin YJ, Chen PC, Chen WS, Lu CL, et al. Association of bone morphogenetic proteins with spinal fusion in ankylosing spondylitis. J Rheumatol. 2010;37(10):2126–32. doi:10.3899/jrheum.100200.
Heiland GR, Appel H, Poddubnyy D, Zwerina J, Hueber A, Haibel H, et al. High level of functional dickkopf-1 predicts protection from syndesmophyte formation in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71(4):572–4. doi:10.1136/annrheumdis-2011-200216.
Daoussis D, Liossis SN, Solomou EE, Tsanaktsi A, Bounia K, Karampetsou M, et al. Evidence that Dkk-1 is dysfunctional in ankylosing spondylitis. Arthritis Rheum. 2010;62(1):150–8. doi:10.1002/art.27231.
Yucong Z, Lu L, Shengfa L, Yongliang Y, Ruguo S, Yikai L. Serum functional dickkopf-1 levels are inversely correlated with radiographic severity of ankylosing spondylitis. Clin Lab. 2014;60(9):1527–31.
Nocturne G, Pavy S, Boudaoud S, Seror R, Goupille P, Chanson P, et al. Increase in dickkopf-1 serum level in recent spondyloarthritis. Data from the DESIR cohort. PLoS One. 2015;10(8):e0134974. doi:10.1371/journal.pone.0134974.
Croes M, Oner FC, van Neerven D, Sabir E, Kruyt MC, Blokhuis TJ, et al. Proinflammatory T cells and IL-17 stimulate osteoblast differentiation. Bone. 2016;84:262–70. doi:10.1016/j.bone.2016.01.010.
Ono T, Okamoto K, Nakashima T, Nitta T, Hori S, Iwakura Y, et al. IL-17-producing gammadelta T cells enhance bone regeneration. Nat Commun. 2016;7:10928. doi:10.1038/ncomms10928.
•• Cortes A, Maksymowych WP, Wordsworth BP, Inman RD, Danoy P, Rahman P, et al. Association study of genes related to bone formation and resorption and the extent of radiographic change in ankylosing spondylitis. Ann Rheum Dis. 2015;74(7):1387–93. doi:10.1136/annrheumdis-2013-204835. First study to link specific genes to disease progression in SpA.
• Nigil Haroon N, Szabo E, Raboud JM, McDonald-Blumer H, Fung L, Josse RG, et al. Alterations of bone mineral density, bone microarchitecture and strength in patients with ankylosing spondylitis: a cross-sectional study using high-resolution peripheral quantitative computerized tomography and finite element analysis. Arthritis Res Ther. 2015;17:377. doi:10.1186/s13075-015-0873-1. A novel approach to asses structural changes in axial spondyloarthritis.
Acknowledgements
B.N obtained an “aspirant” fellowship from the Fund for Scientific Research Flanders (FWO Vlaanderen). Laboratory studies by the authors on the topic are supported by FWO grant G.0946.14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Barbara Neerinckx declares that she has no conflict of interest.
Rik Lories reports grants and personal fees from Pfizer, personal fees from Abbvie, personal fees from Celgene, personal fees from Jansen, personal fees from UCB, grants from Boehringer-Ingelheim, and personal fees from Novartis during the conduct of the study.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Spondyloarthritis
Rights and permissions
About this article
Cite this article
Neerinckx, B., Lories, R.J. Structural Disease Progression in Axial Spondyloarthritis: Still a Cause for Concern?. Curr Rheumatol Rep 19, 14 (2017). https://doi.org/10.1007/s11926-017-0639-7
Published:
DOI: https://doi.org/10.1007/s11926-017-0639-7